Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
While gynecomastia with Risperdal use in males is identified as a rare side effect, some 90 percent of the medication’s users have higher than normal prolactin levels during treatment, according to an article published in the Journal of Clinical Psychiatry and noted on the website drugwatch.com. It is the hormone prolactin that encourages breast tissue growth and is especially problematic in males.
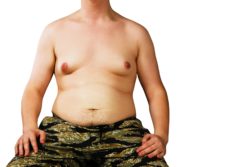
Nearly 1,900 children and adolescents participated in Risperdal’s clinical trials. Gynecomastia with Risperdal occurred in 43 males that were part of this group. Despite this relatively small number, there have reportedly been several hundred boys who have developed breasts while taking Risperdal under doctor’s orders, according to drugwatch.com. The size of the breast tissue growth has varied from small to DD cups—an embarassing and socially-isolating side effect.
What is Risperdal Prescribed to Treat?
WebMD indicates that Risperdal — known generically as Risperidone —is used to treat certain mental illness and mood disorders such as schizophrenia and bipolar disorder. It has also been used to help control irritability associated with autism spectrum disorder.
Although not approved by the U.S. Food and Drug Administration (FDA) for this purpose, physicians often prescribe Risperdal for Attention Deficit Hyperactivity Disorder (ADHD), sleep disorders, anxiety disorders, such as obsessive-compulsive disorder, and clinical depression, according to drugwatch.com.
Are There Early Signs of Gynecomastia with Risperdal Treatment?
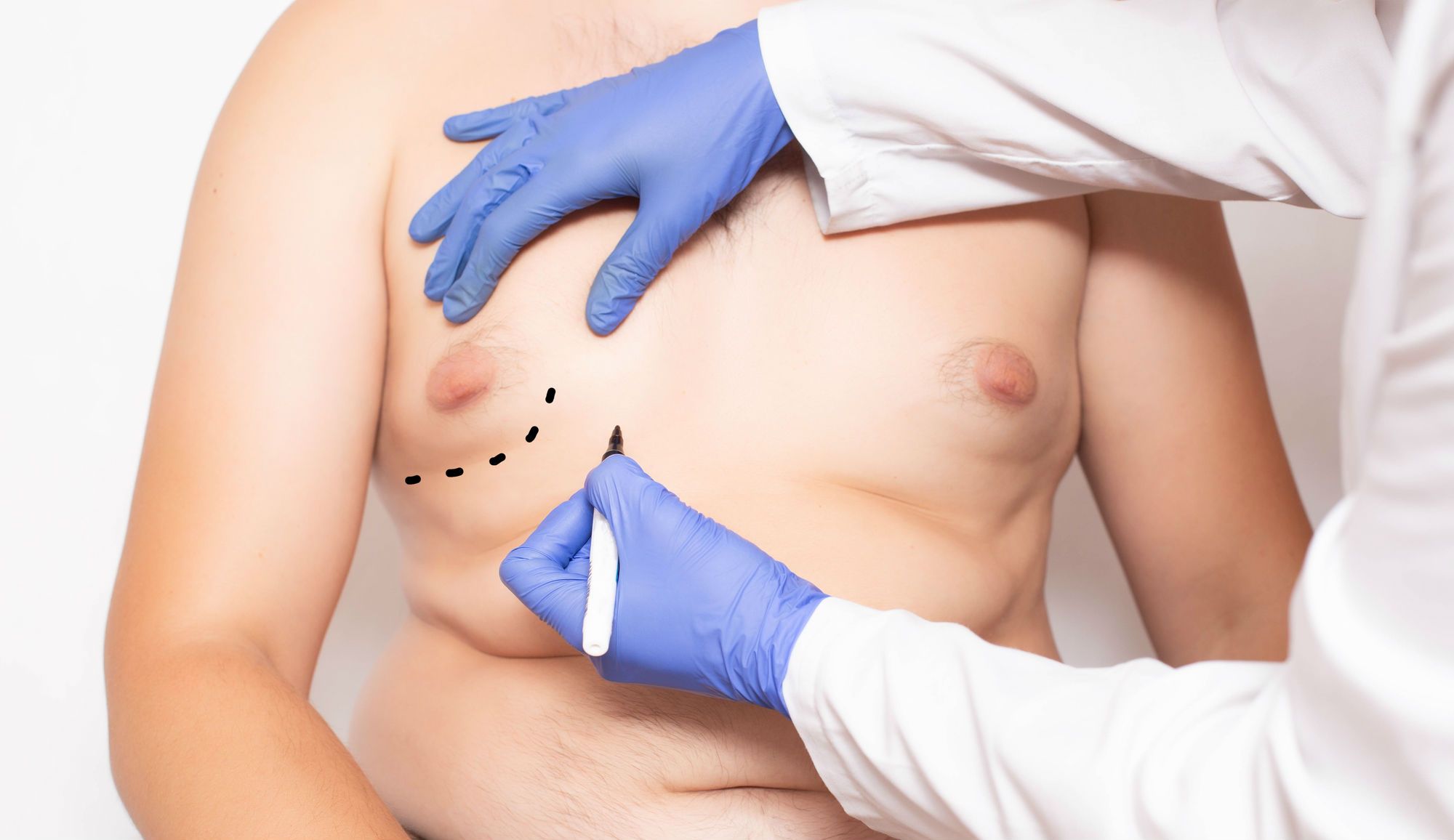
The most common early sign that gynecomastia may be developing with Risperdal treatment is the appearance of a small lump under the nipple on one or both sides of the chest. The lump, which can asymmetrically affect just one side, may be tender to the touch or painful without palpation. The lump may start to gradually enlarge and the nipple could produce discharge or even breast milk. This condition is known as galactorrhea and is related to gynecomastia.
What Causes Gynecomastia?
According to drugwatch.com, prolactin is the force behind the male sex drive, and this hormone is produced in the pituitary gland. Levels of prolactin are brought into check by dopamine—a neurotransmitter produced in the brain. Risperdal has been known to block dopamine and cause prolactin levels to gradually rise in increments to abnormal levels.
In boys and young men, this side effect may cause breast tissue growth and lactation. This can also happen in girls and young women, though it is much less thought of as abnormal unless the patient is quite a bit younger than puberty.
The Mayo Clinic describes the cause of gynecomastia as an imbalance between estrogen and testosterone. Testosterone is thought of as a male hormone and estrogen a female one, but males produce estrogen in small quantities, while females produce a small amount of testosterone. When the delicate balance becomes lopsided toward estrogen, it sets the stage for gynecomastia.
Is There A Treatment for Gynecomastia?
Some success in treating gynecomastia has been achieved by stopping treatment and finding alternative medication for the psychiatric or mood disorder involved. This usually works best in the earliest stages of development or in very modest cases. In other situations, surgery is often performed to remove breast tissue. This option is generally used if breast tissue remains after the patient has been off Risperdal for at least a year.
Have There Been Risperdal Lawsuits?
A Pennsylvania jury found that drugmaker Johnson & Johnson failed to warn a man who suffered Risperdal-related gynecomastia and determined the pharmaceutical giant should pay him an eight-figure award. In October 2019, Johnson & Johnson was ordered to pay $8 billion to settle the lawsuit filed by Nicholas M., a Maryland man who claimed that Risperdal caused him to develop breasts. Nicholas’s lawsuit was the first Risperdal case to result in punitive damages against the drugmaker.
According to Nicholas, he was prescribed the drug at the age of nine to help manage his autism, and was not warned of the possible physical side effects that could occur. The award was eventually reduced to $6.8 billion plus nearly $700,000 in compensatory damages. Johnson & Johnson has characterized the amount as “grossly disproportionate.” The company has stated that it plans to appeal.
In addition to claiming that the pharmaceutical company failed to warn patients of the possibility of developing gynecomastia with Risperdal use, some victims have claimed that the company intentionally covered up the potential side effect, particularly for patients who were prescribed the drug for off-label purposes. In 2013, Reuters reported that Johnson & Johnson agreed to pay $2.2 billion “to end civil and criminal investigations into kickbacks to pharmacists and the marketing of pharmaceuticals for off-label uses.”
More than 13,000 claims against Johnson & Johnson are currently pending regarding Risperdal side effects.
Join a Free Risperdal Side Effects Lawsuit Investigation
If you or your son took Risperdal and experienced any of the following Risperdal side effects listed below, you may qualify to pursue compensation for your injuries:
- Male breast growth
- Man boobs
- Gynecomastia
- Painful breasts
- Nipple pain
- Nipple discharge
See if you qualify by filling out the form on this page for a free case evaluation by a Risperdal gynecomastia attorney.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Risperdal Side Effects Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
Oops! We could not locate your form.