Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
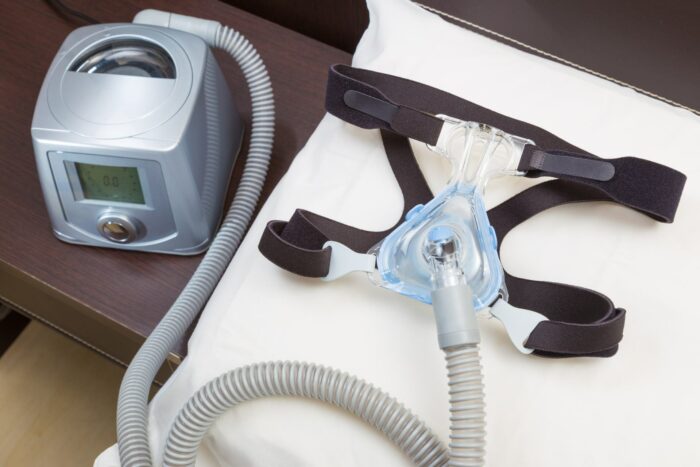
Philips is recalling millions of its CPAP machines, ventilators, and other breathing devices – many used for sleep apnea – due to potential risks of chemical exposure posed by noise-reducing foam in the devices made from polyester-based polyurethane.
Between three and four million of the Bi-Level Positive Airway Pressure (Bi-Level PAP), Continuous Positive Airway Pressure (CPAP), and mechanical ventilator devices are being recalled.
The company has not received any reports of death connected to the sound abatement foam or any reports of impact from chemical emissions, but it has received reports of possible patient impact due to foam degradation, it says in the recall notice.
“The potential risks of particulate exposure include headache, irritation, inflammation, respiratory issues, and possible toxic and carcinogenic effects. The potential risks of chemical exposure due to off-gassing include headache, irritation, hypersensitivity, nausea/vomiting, and possible toxic and carcinogenic effects.”
Despite a low complaint rate (0.03 percent in 2020), testing by the company determined that there were possible risks to users related to this type of foam, including that it might degrade into particles. Philips says in the notice that those particles may enter the device’s air pathway and be ingested or inhaled by the user, and the foam may off-gas certain chemicals.
“The foam degradation may be exacerbated by use of unapproved cleaning methods, such as ozone, and high heat and high humidity environments may also contribute to foam degradation,” the company says.
Frans van Houten, CEO of Royal Philips said the company deeply regrets any concern and inconvenience caused to patients using the affected devices.
“In consultation with the relevant regulatory agencies and in close collaboration with our customers and partners, we are working hard towards a resolution, which includes the deployment of the updated instructions for use and a comprehensive repair and replacement program for the affected devices. Patient safety is at the heart of everything we do at Philips.”
The company is urging patients using BiLevel PAP and CPAP devices to discontinue use and to work with their physician to determine the most appropriate options for continued treatment.
Philips says that patients using life-sustaining mechanical ventilator devices should not stop or alter their prescribed therapy until they have talked to their physician.
“Philips recognizes that alternate ventilator options for therapy may not exist or may be severely limited for patients who require a ventilator for life-sustaining therapy, or in cases where therapy disruption is unacceptable,” the company says in the recall notice.
“In these situations, and at the discretion of the treating clinical team, the benefit of continued usage of these ventilator devices may outweigh the risks identified in the recall notification.”
Philips says that it will replace the current sound abatement foam with a new material and has already begun the preparations, which include getting the relevant regulatory clearances. It says that it aims to address all affected devices in scope of this correction as expeditiously as possible.
“As part of the program, the first-generation DreamStation product families will be modified with a different sound abatement foam and shipped upon receipt of the required regulatory clearances. Philips’ recently launched next-generation CPAP platform, DreamStation 2, is not affected by the issue.”
To support the program, Philips is increasing the production of its DreamStation 2 CPAP devices that are available in the US and selected countries in Europe.
For more information on the recall notification, as well as instructions for customers, users and physicians, affected parties may contact their local Philips representative or visit www.philips.com/SRC-updateExternal Link Disclaimer.
Have you used a breathing device manufactured by Philips? How do you feel about this recall? Let us know in the comments section or join a CPAP class action lawsuit investigation!
Don’t Miss Out!
Check out our list of Class Action Lawsuits and Class Action Settlements you may qualify to join!
Read About More Class Action Lawsuits & Class Action Settlements:
- Consumer Protection Agency Announces New Rules for Dangerous Infant Sleepers
- Do You Qualify: Asbestos Cancer | Mesothelioma and Lung Cancer Lawsuit Claim Review
- Recall Check: Fisher-Price Recalls Gliders After Four Infant Deaths
- Do You Qualify: Surgical Staples Complications and Recall Lawsuit Investigation















117 thoughts onRecall Check: Philips Recalls Millions of CPAP and Ventilators Over Fears of Chemical Exposure, Carcinogenic Effects
I have been using three diff sleep apnea for the past 15 years
I have been coughing continuously after i used the above machines, besides i am having severe headache, which stretches to ear, teeth with severe pain do not have pain free sleep
i am 82 years old i causes COPD symptoms to me
i am having cough with phlegm in the morning hours
i also feel lung congestions and tightness of the chest
and feeling smell of the foreign substances
i also have respiratory issues including wheezing etc.
i am seeing my PCP to get prescription for CT scan of head and chest, throat to ascertain the condition and to find out what causes severe headache. I never had headache in my lifetime.
i shall send you copies of the medical investigations for your review and to notify manufacturers of Sleep apnea machine.
Add me I have the cpap and have not even been notified about the recall
Please add me I have a phillips cpap machine, but haven’t been warned about the recall, had to get the machine fixed and they charged me 300.00 to have it fixed because they said water was in the machine and it messed up.
Add Me, I’ve used the Phillips C-Pap for apx 9 years.
I have two of the recalled machines which I have used for about 9 years. My shortness of breath may well be explained by this introduction of toxic particles. It frustrates me that with no solution offered, I had to purchase a new machine of another brand. No wonder the sleepstation 1 became so cheap and sleepstation 2 shows up all in time for the recall. Not even a discount offered on the replacement version (though I wouldn’t trust it). A responsible company should have at least offered expedient solutions!!
I developed a terrible polyster allergy resulting in me getting rid of my clothes, couch and linens. Anything that could possible contain polyster. I had no clue that this could possible be the source of my aliment. But it would all make sense now since it all start around the time I got my dream machine around June 2018. I have had 3 chest infections and 2 sinus infection since then and have start using a inhaler.
i FULLY agree with you i have similar problems as a result of
use of sleep apnea machines three diff models
u can communicate to me further course of action against mfr
I have been using the cpap dream station for four years. I have had a constant sinus infection that will not go away with antibiotics. I nonstop cough and no I need sinus surgery for a deviated septum caused by an concha bollusa.
I have two Phillips Respironics CPAP machines, original DreamStation and DreamStation GO.
Everyone I call says we are working on it?? It has been at least ten days. Do we wait until people die? Unbelievable! Lack of sleep triggers seizures besides having sleep apnea.
Please add me.I was diagnosed with copd last year and horrible headache s.I have a dream station.