Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
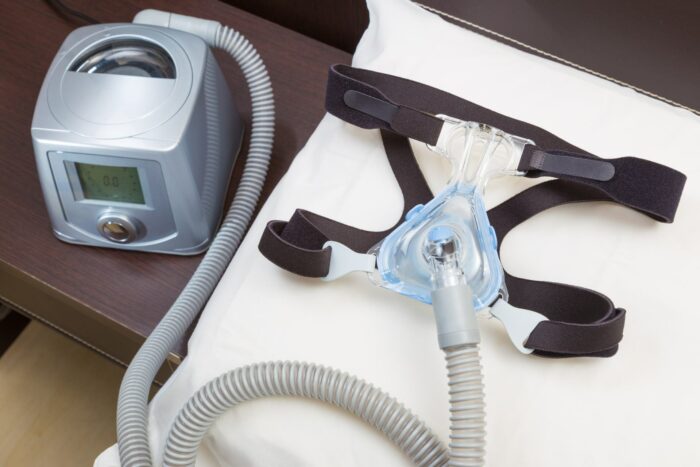
Philips is recalling millions of its CPAP machines, ventilators, and other breathing devices – many used for sleep apnea – due to potential risks of chemical exposure posed by noise-reducing foam in the devices made from polyester-based polyurethane.
Between three and four million of the Bi-Level Positive Airway Pressure (Bi-Level PAP), Continuous Positive Airway Pressure (CPAP), and mechanical ventilator devices are being recalled.
The company has not received any reports of death connected to the sound abatement foam or any reports of impact from chemical emissions, but it has received reports of possible patient impact due to foam degradation, it says in the recall notice.
“The potential risks of particulate exposure include headache, irritation, inflammation, respiratory issues, and possible toxic and carcinogenic effects. The potential risks of chemical exposure due to off-gassing include headache, irritation, hypersensitivity, nausea/vomiting, and possible toxic and carcinogenic effects.”
Despite a low complaint rate (0.03 percent in 2020), testing by the company determined that there were possible risks to users related to this type of foam, including that it might degrade into particles. Philips says in the notice that those particles may enter the device’s air pathway and be ingested or inhaled by the user, and the foam may off-gas certain chemicals.
“The foam degradation may be exacerbated by use of unapproved cleaning methods, such as ozone, and high heat and high humidity environments may also contribute to foam degradation,” the company says.
Frans van Houten, CEO of Royal Philips said the company deeply regrets any concern and inconvenience caused to patients using the affected devices.
“In consultation with the relevant regulatory agencies and in close collaboration with our customers and partners, we are working hard towards a resolution, which includes the deployment of the updated instructions for use and a comprehensive repair and replacement program for the affected devices. Patient safety is at the heart of everything we do at Philips.”
The company is urging patients using BiLevel PAP and CPAP devices to discontinue use and to work with their physician to determine the most appropriate options for continued treatment.
Philips says that patients using life-sustaining mechanical ventilator devices should not stop or alter their prescribed therapy until they have talked to their physician.
“Philips recognizes that alternate ventilator options for therapy may not exist or may be severely limited for patients who require a ventilator for life-sustaining therapy, or in cases where therapy disruption is unacceptable,” the company says in the recall notice.
“In these situations, and at the discretion of the treating clinical team, the benefit of continued usage of these ventilator devices may outweigh the risks identified in the recall notification.”
Philips says that it will replace the current sound abatement foam with a new material and has already begun the preparations, which include getting the relevant regulatory clearances. It says that it aims to address all affected devices in scope of this correction as expeditiously as possible.
“As part of the program, the first-generation DreamStation product families will be modified with a different sound abatement foam and shipped upon receipt of the required regulatory clearances. Philips’ recently launched next-generation CPAP platform, DreamStation 2, is not affected by the issue.”
To support the program, Philips is increasing the production of its DreamStation 2 CPAP devices that are available in the US and selected countries in Europe.
For more information on the recall notification, as well as instructions for customers, users and physicians, affected parties may contact their local Philips representative or visit www.philips.com/SRC-updateExternal Link Disclaimer.
Have you used a breathing device manufactured by Philips? How do you feel about this recall? Let us know in the comments section or join a CPAP class action lawsuit investigation!
Don’t Miss Out!
Check out our list of Class Action Lawsuits and Class Action Settlements you may qualify to join!
Read About More Class Action Lawsuits & Class Action Settlements:
- Consumer Protection Agency Announces New Rules for Dangerous Infant Sleepers
- Do You Qualify: Asbestos Cancer | Mesothelioma and Lung Cancer Lawsuit Claim Review
- Recall Check: Fisher-Price Recalls Gliders After Four Infant Deaths
- Do You Qualify: Surgical Staples Complications and Recall Lawsuit Investigation















117 thoughts onRecall Check: Philips Recalls Millions of CPAP and Ventilators Over Fears of Chemical Exposure, Carcinogenic Effects
My husband been using the dream station , got through VA. Didn’t know about recall . Until someone let the cat out of the bag! The VA said go ahead and still use it , we looked it up and it was one of the recalls . My husband has gone without treatment for his sleep apnea ? Year in a half , close it’s been a long time . The VA couldn’t get any , shortage ! Still don’t have any c-paps ! And to think he was thinking and trusting in the machine , unbelievable
Add me I have the cpap and have not even been notified about the recall
Nobody has contacted me to tell me there is any recall on my Phillips Respironic cpap machine. I developed severe sinus infections and headaches every time I used my cpap. I tried to file a claim with this class action suite but was told that if I used to smoke cigarettes for at least 15 years that I could not qualify for any class action claims. I quit smoking over 20 years ago and have no problems with my lungs or any other issues. I used to use a Phillips bi- level cpap before I was put on the regular cpap machine. I was not able to use either machine for very long before I started getting severe sinus and headache issues. I made sure I cleaned my hoses and machine very well before using it but still would wake up with problems.
I quit using my Phillips CPAP machine because of sinus infections and headaches, also shortness of breath
They’ve done nothing to help me with the defective equipment they sold. I responded months ago and they just keep sitting on their hands looking at the ceiling hoping it all goes away. They need to put their tea down splash some cold water on their face and get ready for the court room the bunch of lazy buggers.
I had a Phillips Respironic cpap machine and one day the smell in my lungs in the morning was foul. I then checked on google if there was a reason and then saw it had been recalled. I stopped using it and used a travel cpap but my lungs had that smell that would not go away and it appeared that it infiltrated my travel cpap tried a second cpap and same thing. I then stopped using a cpap and was sleeping sitting up with fear of stop breathing. I proceeded to order a new travel cpap as the regular cpap machines are not yet available due to the failure if mist cpap machines. At thus time the foul smell is still coming thru my skin . I asked my doctor and they seem to not know what to do?? I fear that my insides seem to be poisoned by that Respironic Phillips cpap. My bedding when I get has that foul smell from my body and no matter how many time I shower, or change my bedding it smells when I get up. Can someone let me know if there is a solution and does anyone else have that issue??
Es increíble tanto hermetismo no a todas la personas se les a informado. Es una falta de respeto que tengamos que invertir en otro equipo simplemente por que no son capases de sustituirlo para mitigar los daños. No todos tienen el dinero y para colmo hay escases de estos lo que provocará el alza en el costo.
Poniendo en riesgo nuestra salud y sobre todo nuestras vidas.
At the least, stated in their warranty contract. If your phillips dreamstation 1 was still under warranty you should have been shipped a dreamstation 2 immediately just because after recalling it it became defective which is grounds for replacement not just ignoring their paying customers, even more so because of the recall. They will pay or replace, not just repair devices still under warranty by law. I have had 2 Phillips auto cpap machines in 10 years and had a MRI done 1 1/2 years ago showing complete sinus cavities blockage with severe inflamation after using the dreamstation 1 for 1 year.
We were not notified of the recall. I found out through word-of-mouth. Why isn’t this public yet? Why aren’t distributors NOTIFYING people??
Que va hacer Phillips para los que tengan dicha maquina.