Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
The U.S. Food and Drug Administration (FDA) approved the gout medication in 2009 with a label warning about the possibility of Uloric heart attack, stroke, and cardiac ischemia.
The agency also demanded that the drug’s manufacturer, Chicago-based Taketa Pharmaceuticals, do a post-market follow-up study to verify these initially identified risks.
In a recent safety-related press release in November 2017, the FDA indicated that of 6,000 patients involved in the required follow-up study, half were placed on Uloric (identified by its generic name febuxostat) and half were prescribed allopurinol. Allopurinol is a competing treatment drug for gout. Uloric did not reveal an overall increase in the risk of death from Uloric heart attack, stroke, or risk of non-deadly cardiac events when compared to this competitor.
The FDA did say, however, that when the results of this study were evaluated closely and separately from the allopurinol control group, it did show an increased risk of death occurring from Uloric heart attack, critical heart ischemia—a condition of reduced blood flow and resultant oxygen to the cardiac muscle—and stroke.
In addition to indicating an increased risk of Uloric heart attack and other serious heart events when evaluated separately, the study revealed a statistically significant risk of dying from all causes while taking the drug.
Because of this study, the FDA will be continuing to keep its eye on adverse event reports regarding this drug. In the meantime, it has encouraged medical professionals to be careful and consider all risk factors such as obesity, smoking, the presence of Diabetes or other concurrent illnesses when deciding upon a course of treatment for gout with patients.
At this time, the label on febuxostat will remain as it was from its initial 2009 approval—warning consumers of the potential of a Uloric heart attack while on the drug.
What is Gout?
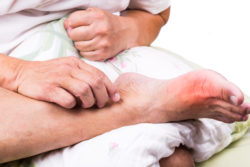
Gout is an arthritic condition usually treated by specialty doctors known as rheumatologists. It is caused by a build-up of uric acid in the body and reveals itself through painful inflammation and redness in one or more joints. The uric acid build-up forms crystals in the affected joint which is, although not always, one of the big toes. While it may start in one joint, it can spread to others and cause widespread joint damage.
Gout attacks men more often than women and typically come on after the mid-forties and beyond. Statistically speaking, women catch up with men in developing gout symptoms after menopause. Gout has been often referred to as the disease of ‘rich-living’ as some of the risk factors include a diet filled with red meat and organ meat as well as alcohol.
Consumption of foods containing large amounts of high-fructose corn syrup can also contribute to its onset. It can also happen, however, because of other health conditions such as kidney disease, diabetes, high blood pressure and heart disease or familial genetic tendencies.
In general, Uloric lawsuits are filed individually by each plaintiff and are not class actions.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual Uloric lawsuit or Uloric class action lawsuit is best for you. Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Uloric Class Action Lawsuit Investigation
If you suffered from a serious side effect or a loved one died while taking Uloric, you may have a legal claim. See if you qualify to pursue compensation and join a free Uloric lawsuit investigation by submitting your information for a free case evaluation.
An attorney will contact you if you qualify to discuss the details of your potential case.
Please Note: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client, if you qualify, or getting you dropped as a client.
Oops! We could not locate your form.












