Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
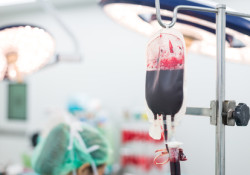
The plaintiffs, hailing from Arizona, Texas, and Oklahoma, generally allege personal injury and loss traceable to excessive bleeding that occurred during a course of treatment with the anticoagulant drug Xarelto.
Plaintiffs Rita S., John R. and Rose R. all allege they each suffered some form of internal bleeding as a direct result of taking Xarelto.
Plaintiffs Carlos M. and Irene R., the respective spouses of plaintiffs Rita and John, are each bringing a claim for loss of consortium. This type of claim seeks compensation for the loss of their spouse’s “support, companionship, services, society, love and affection” that resulted from Xarelto complications.
In another plaintiff’s case, Xarelto complications allegedly ended in the patient’s death. The Xarelto lawsuit includes claims for survival and wrongful death brought by plaintiff Ginger T. on behalf of the late Andy C.
Andy died allegedly as a result of severe internal bleeding after taking Xarelto. Ginger now serves as the executor of Andy’s estate.
Xarelto Side Effects
Xarelto is a relatively new anticoagulant drug, one of a group of such drugs that recently entered a market that had been dominated for decades by warfarin. Sales of Xarelto in the U.S. began after initial FDA approval in 2011.
Like many other anticoagulants, Xarelto is prescribed to help reduce the risk of stroke and systemic embolism in patients with atrial fibrillation. It’s also used to prevent other blood-clot related injuries like pulmonary embolism and deep vein thrombosis in patients who are particularly susceptible to those conditions.
Among other arguments, plaintiffs take issue with some of the main marketing points promoted by Xarelto’s manufacturers, Janssen.
The defendant manufacturers have promoted what they profess as the “Xarelto Difference,” a set of advantages the new medication supposedly has over warfarin.
They say that Xarelto users can avoid the frequent blood tests and dosage adjustments associated with warfarin, and that they can also enjoy convenient once-daily dosing.
Plaintiffs, however, say that the evidence from clinical trials show that Xarelto is safer and more effective in conjunction with regular blood monitoring and dose adjustments.
They also cite comments from FDA reviewers saying that spreading out the dosing over two doses per day would eliminate the peaks and dips in blood levels that were reported during clinical trials.
The plaintiffs also argue that the manufacturers overpromoted Xarelto. They say that during 2013 alone, the defendants spent at least $11 million on advertisements in journals, targeting both patients and physicians in the US. Sales that year reportedly exceeded $1 billion, earning the drug “blockbuster” status.
Plaintiffs accuse the defendants of overstating Xarelto’s efficacy and downplaying the dangers of Xarelto side effects in this promotional effort.
They cite an enforcement letter from the FDA ordering the defendants to cease production of some of the promotional material because it “minimizes the risks associated with Xarelto and makes a misleading claim” about the need for dose adjustments.
Plaintiffs allege the “Xarelto Difference,” far from offering patients any benefit, was in fact “a marketing campaign based on flawed science.”
The Xarelto Lawsuit is Case No. 2:16-cv-6546 in the U.S. District Court for the Eastern District of Louisiana.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The Xarelto attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or Xarelto class action lawsuit is best for you. [In general, Xarelto lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
Get Help – It’s Free
Join a Free Xarelto Class Action Lawsuit Investigation
If you or a loved one took Xarelto (rivaroxaban) and suffered injuries such as uncontrollable internal bleeding, gastrointestinal bleeding, hemorrhaging, deep vein thrombosis or pulmonary embolism, you may have a legal claim. See if you qualify by filling out the short form below.
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Please Note: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client, if you qualify, or getting you dropped as a client.
Oops! We could not locate your form.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.












