Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
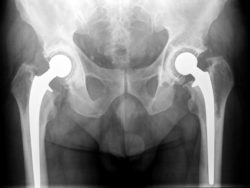
Plaintiff Anita P. claims that she had the Biomet Magnum M2a hip system installed to replace her left hip in 2008, and had the system installed to replace her right hip in 2012.
She claims that the hip system was defective, caused the component metal to fret into her body, requiring a revision surgery on her right hip in 2016. She claims that the manufacturers knew that the hip system was dangerous to patients, but continued to release the device into the stream of commerce.
She seeks damages to compensate for the physical and emotional injury. She states that risk of complication and hip dislocation increase greatly when a patient has to have a revision surgery, and the physical injury incurred from repairing the damage done by the Biomet Magnum M2a is significant.
According to Anita, the Biomet Magnum M2a system possesses a manufacturing defect and was not adequately tested before being used on patients. Allegedly, in some patients, the components of the hip system loosen prematurely and more than the components of the average hip system, requiring revision surgery.
In other cases, the component parts of the hip system allegedly wear incorrectly, causing chromium and cobalt to wear and corrode from multiple parts of the device into a patient’s body. This, in turn, can cause infection, fluid accumulation, and tissue death in the body around the device. The body often then rejects the impact as a way of healing itself.
Patients have reported that they experience the following symptoms of their body rejecting the M2a hip system as a result of its alleged defects:
- pain
- looseness
- dislocation
- squeaking and popping sounds
According to Anita, the Biomet Magnum M2a system was not sufficiently tested before being released into the stream of commerce and used in patients. Allegedly, the Food and Drug Administration never approved the hip system to be safe or effective.
Anita claims that Biomet marketed the Biomet Magnum M2a as safe and effective despite its lack of FDA approval. Allegedly, Biomet representatives held meetings with orthopedic surgeons around the country, touting the system’s “excellent track record,” as well as its “low and acceptable failure rate.”
According to the Biomet defective hip implant lawsuit, there have been over 350 reports of the Biomet Magnum M2a failing and injuring patients. According to the lawsuits, these reports started as early as 2004. Allegedly, Biomet continued to defend the quality of and promote the hip system in light of these patient reports. They allegedly actively concealed the reports of failure from both patients and medical professionals.
Anita claims that the company should have ceased the sale of the Biomet Magnum M2a in light of the patient reports, and alerted patients and medical professionals to the risks associated with the product’s alleged defects.
The Biomet Magnum M2a Defect Lawsuit is USDC IN/ND Case No. 3:18-cv-00236, in the U.S. District Court for the Northern District of Indiana, South Bend Division.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The hip implant attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. [In general, metal hip implant lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free DePuy, Zimmer Hip Replacement Lawsuit Investigation
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Please Note: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client, if you qualify, or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.
Oops! We could not locate your form.












