Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
Australian regulators have banned three types of textured breast implants due to risks of a rare breast implant cancer.
The Australian Therapeutic Goods Administration (TGA) has removed three textured implant products off of the Register of Therapeutic Goods: Polytech (JT Medical) Sublime Line Microthane Silicone; Polytech 4Two Line Single Lumen Micro Polyurethane Silicone implants; and Euro Implants (Eurosilicone SAS) Cristaline Paragel Cohesive Gel Implant. As a result of these actions, the products cannot be supplied, imported, or exported from Australia unless special authorization is given by authorities.
The recent move against textured implants was a step up from a October 2019 suspension of silicone and polyurethane implants by the TGA. Eight types of “macro” and “micro” textured implants were recalled and suspended while the authorities reviewed “a number of safety and performance concerns.” After this review, the TGA reportedly decided to ban the products permanently.
The ban stems from concerns that textured implants are associated with a rare form of breast cancer. Due to these risks, medical professionals support the TGA’s recent decisive action.
“The Australian Society of Plastic Surgeons has been working with the TGA to minimize the risk of harm to Australian women and Australian specialists plastic surgeons are contributing to global research into this rare cancer,” Dr. Dan Kennedy, President of the Australian Society of Plastic Surgeons, said in a statement according to the Sydney Morning Herald.
“BIA ALCL is a rare cancer and can be successfully treated if detected early. We would emphasize that cancellation of the implants does not mean that implants that have previously been inserted should be removed if there are no symptoms to indicate ALCL.”
Textured Breast Implants: Overview
There are several types of breast implants available on the market for use in plastic surgery. Saline, silicone, structured, “gummy bear,” round, and other classifications are used to describe these implants based on their shape, materials, and textures.
Implants may also be described as “textured.” As opposed to smooth implants which have no external texture, textured breast implants have a tangible texture on their outer surface. This helps scar tissue to stick to the implants during the healing process – reducing the risk of a tight scar capsule and even helping to reduce potential movement of implants within the breast.
 Breast Implants & Cancer
Breast Implants & Cancer
Although there are benefits to textured implants, these products have recently come under scrutiny for a potential link to a form of cancer known as breast implant-associated anaplastic large cell lymphoma (BIA ALCL).
Despite its name, BIA ALCL is not actually a type of breast cancer. Instead, the condition is a type of immune system cancer falling under the non-Hodgkin’s lymphoma category. BIA ALCL is unique because it develops in the scar tissue capsule which forms around the breast implant during the healing process.
Symptoms of BIA ALCL can vary, but often include swelling in the breast implant along with the pain and the feeling of a mass. Upon evaluation, doctors may also see signs of fluid collection around the breast implant or scar capsule contracture. These symptoms may not show up until years after a breast implant is initially implanted and healed.
Luckily, if the cancer is caught early it is highly treatable. If cancerous cells or tumors are concentrated in the tissue and fluid near the implant, it can be removed and, if necessary, treated with chemotherapy and radiation therapy. However, there is the risk that the cancer will spread to other parts of the body – including to the nearby lymph nodes found in the armpit area.
BIA ALCL was first identified by the U.S. Food and Drug Administration (FDA) in 2011 but has become more of a prominent concern in recent years. As more research is uncovered, it has become clear that BIA ALCL risk may increase with the use of textured breast implants.
In 2019, the FDA acted on this evidence when they called for breast implant manufacturer Allergan to recall several of their textured implant products. According to the FDA’s press release, 481 out of 573 unique cases of BIA ALCL known to the agency at the time were associated with the company’s implants. Even worse, 12 out of 13 patient deaths were associated with Allergan implants when the identity of the manufacturer was known.
Although the idea of developing any form of cancer is frightening, most health professionals do not recommend having breast implants removed simply because of concerns surrounding BIA ALCL. Instead, patients are encouraged to speak to their doctor if they have any symptoms of BIA ALCL or other changes.
Join a Free Breast Implants Side Effects Lawsuit Investigation
You may qualify for this breast implant investigation under these circumstances:
- You were implanted with BIOCELLE or Natrelle textured breast implants;
- You were diagnosed with Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA ALCL); or
- You have had revision surgery after learning about this cancer risk.
Fill out the form on this page for a free case evaluation by a breast implants injury attorney.
This article is not legal advice. It is presented
for informational purposes only.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Breast Implants Side Effects Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.


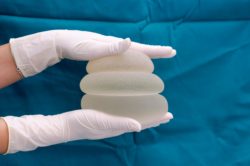 Breast Implants & Cancer
Breast Implants & Cancer