Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
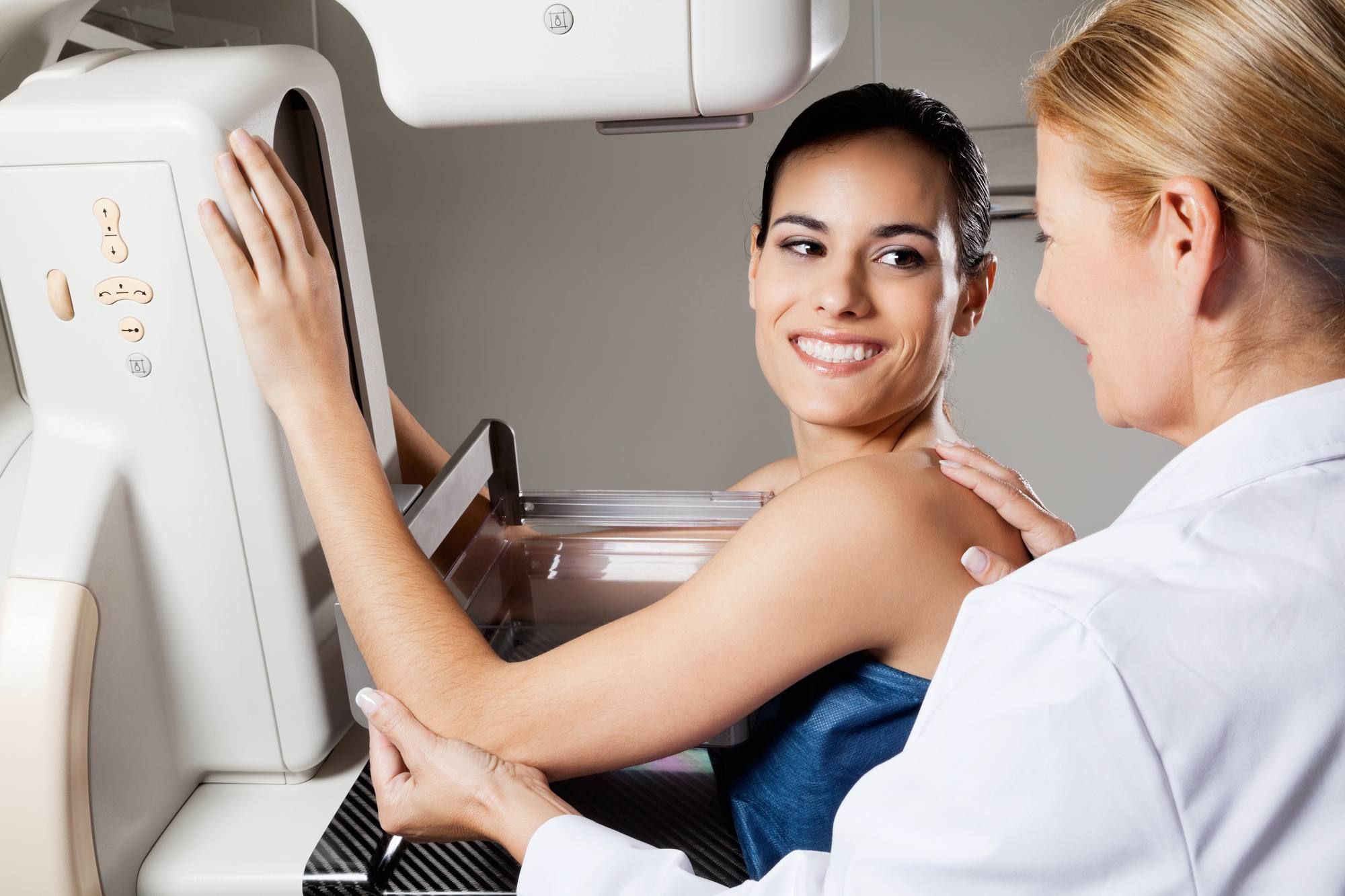
BIA-ALCL is a rare form of lymphoma that starts in the fibrous scar tissue and accompanying fluid surrounding a breast implant. In most cases, this breast implant associated cancer has been found in women with breast implants with textured surfaces.
Allergan recently announced a voluntary recall of its textured breast implants. Dr. Ewa Timek of Advanced Plastic Surgery in Grand Rapids, Mich. told a Michigan Fox affiliate that this is because BIA-ALCL is seen six times more often in women with Allergan’s Biocell textured implants than in those who received implants from other manufacturers.
Symptoms of Breast Implant Illness
Anyone who has received textured breast implants should be wary of these symptoms:
- Breast pain.
- Swelling around the implant area.
- Lumps in the breast or armpit.
- Changes in breast appearance.
- Fluid retention in breasts.
- Skin rash.
- Hardening of the breast.
According to the American Society of Plastic Surgeons, BIA-ALCL is curable if detected early.
Most cases of BIA-ALCL occur years after the breast implants have been implanted. Currently, the FDA is not recommending the removal of breast implants in patients who are not exhibiting symptoms of breast implant illness.
The FDA initially suspected a potential link between breast implants and BIA-ALCL in 2011, but so few cases were reported that the agency was unable to pinpoint specific factors associated with the increased risk.
In 2016, the World Health Organization (WHO) designated BIA-ALCL as a T-cell lymphoma that could develop after a patient receives breast implants. No worldwide database of breast implant procedures exists, which has made it difficult for the WHO to link the cases.
The type of texture on implants’ outer covering appears to be the risk factor for developing BIA-ALCL. The FDA says whether an implant is filled with saline or silicone has not been seen as a major predictor of who will develop cancer, but there is not enough data to make a determination one way or the other.
Agency Releases Breast Implant Illness Report
Fierce Biotech reports that the FDA has issued a publication with new information about the side effects of breast implants and how many patients have suffered from symptoms of breast implant illness. According to the agency, between July 7, 2019 and Jan. 5, 2020, an additional 160 cases of breast implant illness were reported. In addition, three deaths attributed to the condition were reported.
“While the FDA doesn’t have definitive evidence demonstrating breast implants cause these symptoms, the current evidence supports that some patients experience systemic symptoms that may resolve when their breast implants are removed,” said an FDA spokesperson at the time of the report.
Fierce Biotech notes that the additional cases bring the total number of breast implant illness cases reported to the FDA to over 700 and the total number of deaths to 36. Reportedly, many of these cases are attributed to Allergan breast implants – over 600, according to the new publication.
“The FDA has been diligently monitoring adverse events associated with breast implants for decades and has been working to better understand the quality of life and satisfaction a breast reconstruction patient may experience in order to refine our evaluation of breast implant benefits and risks,” said an FDA spokesperson.
However, the new cases reported to the FDA represents a “steep jump,” says Fierce Biotech, noting that just over 1,000 reports of BIA-ALCL were made in the decade prior to 2018.
Join a Free Breast Implants Side Effects Lawsuit Investigation
You may qualify for this breast implant investigation under the following circumstances:
- You were implanted with textured breast implants;
- You were diagnosed with Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA ALCL); and/or
- You’ve suffered from any illness you believe is related to the implants.
Fill out the form on this page for a free case evaluation by a breast implants injury attorney.
This article is not legal advice. It is presented
for informational purposes only.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Breast Implants Side Effects Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.