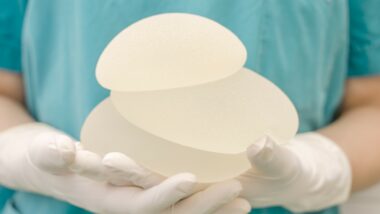
ALCL stands for anaplastic large cell lymphoma. In February 2019, the U.S. Food and Drug Administration warned people that some breast implants, especially textured ones, may be linked to this type of cancer.
Lymphoma Research Foundation notes that ALCL can initially appear in the skin, lymph nodes, or in the organs throughout the body.
When it is associated with breast implants, anaplastic large cell lymphoma is also known as breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). The link between breast implants and this kind of cancer was first identified in 2011. Now the FDA has begun looking further into the issue.
Patients who have had breast implants, especially textured implants, may wonder if they are at risk for ALCL. Some patients may wonder if there is a blood test for ALCL. There are some symptoms that may be associated with ALCL and tests that can indicate if a person has the cancer.
How Is ALCL Linked to Breast Implants?
BIA-ALCL is usually linked to textured breast implants. Textured breast implants are just one of a number of types of implants used in breast augmentation surgery.
The surface of a textured implant is much like sandpaper, and the texture is supposed to prevent the implant from moving around in a person’s chest. This is supposed to prevent the implant from looking misshapen. The texture is also supposed to help scar tissue form around the implant, which prevents the implant from moving.
However, there are drawbacks to different types of breast implants — some patients say that textured implants can feel hard and leave a patient with unnatural feeling breasts.
An issue of greater concern, however, is the possible health effects of textured implants. The texture of the implant does indeed encourage scarring. Experts say that cancer can develop within the scar capsule that forms around the breast implant during the healing process.
How Do I Know if I Am at Risk for ALCL?
The FDA says that the risk for BIA-ALCL is small, but the data reportedly does suggest that most of the cases of BIA-ALCL have occurred in patients who have textured implants. A recent South Korea study finds the same.
What Are the Symptoms of ALCL?
Some symptoms of ALCL include developing a spontaneous fluid collection in the breast. This can develop many months or years after the breast implant was placed. Patients may also experience redness and swelling of the breast around an implant that is not the result of an infection.
Less commonly, patients who have had had breast implants implanted and suffer from ALCL may experience a contraction of the scar tissue capsule around the breast implant. MD Anderson Cancer Center notes that these symptoms are not necessarily caused by ALCL, and could be the result of other health problems.
Is There a Blood Test for ALCL?
ALCL is not commonly diagnosed with a blood test. Instead, the Macmillan Cancer Support organization says that the most common test for ALCL is to remove a sample of an enlarged lymph node and test it for the presence of lymphoma cells. There are other tests and scans to determine the presence of the disease.

- a needle biopsy
- drainage of capsule fluid
- ultrasounds
- CAT scans
- MRI
- other imaging scans
The first step in diagnosing ALCL is to find the first signs of the condition. Web MD explains that doctors will first ask if a patient is experiencing several symptoms. They may ask if the person is experiencing pain, and if so, where it is located. The doctor may also inquire about the person’s appetite, weight loss, or fatigue. The doctor may also ask if the patient has observed any skin bumps or swollen glands. If the patient has identified any of these symptoms, the physician will likely ask when the symptoms began.
Web MD goes on to explain that after identifying symptoms that point to ALCL, a physician may take a biopsy from a lymph node. This may involve removing the whole lymph node, or just a piece of it. Happily, it is a procedure that does not require the patient to stay overnight in a hospital.
This biopsy may be done by making a cut in the skin to remove the lymph node sample or may be done using a needle. The sample is then examined under a microscope to see if any cancer cells are present.
The Lymphoma Research Foundation explains that blood tests are used largely to determine how serious the ALCL is and if the disease has spread into other parts of the body. Blood tests are used to gain more information about an ALCL patient’s condition. Other tests that are also used to gain more information about the patient’s condition include a bone marrow biopsy, a CT scan, and other tests.
Though these options may complicate the question of “is there is blood test for ALCL,” they can provide valuable insight into whether or not a person may have the disease.
FDA Releases New Report on Breast Implant Illnesses
Fierce Biotech reports that the FDA has published new data on the side effects of breast implants, including BIA-ALCL. According to the FDA, there were an additional 160 cases of the illness and three deaths reported between July 7, 2019 and Jan. 5, 2020.
“The FDA has been diligently monitoring adverse events associated with breast implants for decades and has been working to better understand the quality of life and satisfaction a breast reconstruction patient may experience in order to refine our evaluation of breast implant benefits and risks,” said a spokesperson for the agency.
The additional cases reportedly bring the total number of cases reported to the FDA to 733. Thirty-six people have died of the disease. According to the FDA report, 620 of these cases are attributed to Allergan breast implants.
In addition, reports of breast implant illness-related symptoms increased as well, with the FDA accounting for nearly 2,500 reports in a one-year period between 2018 and 2019.
Fierce Biotech says that the rate of new reports is a “steep jump” from previously reported cases of BIA-ALCL which totaled 1,080 reports spanning a decade between 2008 and 2018.
“While the FDA doesn’t have definitive evidence demonstrating breast implants cause these symptoms, the current evidence supports that some patients experience systemic symptoms that may resolve when their breast implants are removed,” stated the agency.
Join a Free Breast Implants Side Effects Lawsuit Investigation
You may qualify for this breast implant investigation under the following circumstances:
- You were implanted with textured breast implants;
- You were diagnosed with Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA ALCL); and/or
- You’ve suffered from any illness you believe is related to the implants.
Fill out the form on this page for a free case evaluation by a breast implants injury attorney.
This article is not legal advice. It is presented
for informational purposes only.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Breast Implants Side Effects Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
[email protected].