Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
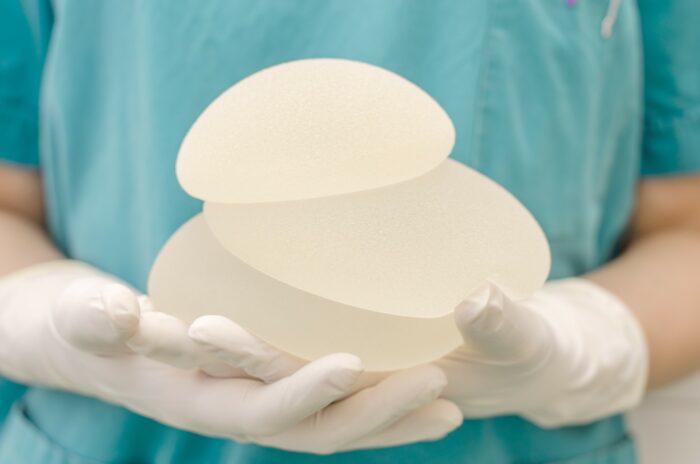
Breast implant maker Allergan can’t escape a class action lawsuit alleging its implants cause cancer, according to a decision in New Jersey state court.
In August 2020, Allergan tried to dismiss consolidated claims which linked its breast implants with cancer, arguing that certain plaintiff claims call for regulations stricter than those imposed by the U.S. Food and Drug Administration. The company also challenged claims made under the federal Food, Drug, and Cosmetics Act (FDCA), noting that plaintiffs failed to accompany these claims with allegations under a parallel New Jersey law. Without these claims, FDCA claims in New Jersey state court cannot survive preemption, the company argued.
In a March 2021 ruling, New Jersey Judge Rachelle L. Harz partially agreed with Allergan’s arguments. Judge Harz dismissed claims of failure to warn and claims surrounding the FDA’s premarket approval process of Allergan implants.
However, breast implant maker Allergan was not entirely successful in its dismissal motion. Judge Harz refused to completely dismiss claims in the multicounty litigation and allowed several claims to move forward. Preserved claims include: strict liability, negligent failure to warn, negligent design defect, manufacturing defect, breach of express warranty, and consumer fraud. By preserving these claims, Judge Harz allows plaintiffs to move forward with their lawsuits.
According to plaintiffs in the case, Allergan failed to warn that their breast implants carried an increased risk of cancer. Specifically, Allergan implants can allegedly cause breast implant-associated anaplastic large cell lymphoma (BIA ALCL).
BIA ALCL is a type of non-Hodgkin’s lymphoma which forms in the scar tissue capsule around a breast implant. The condition is fairly rare, with less than 1,000 cases recorded globally. However, a large portion of these cases are linked to Allergan implants, according to an announcement from the U.S. Food and Drug Administration (FDA).
On July 2019, the FDA revealed that Allergan implants were associated with 481 cases of BIA ALCL out of 573 total cases. Similarly, Allergan implants were reported in 12 BIA ALCL deaths out of 13 deaths in which implant manufacturers were identified.
As a result of this data, the FDA urged Allergan to recall its textured breast implants. The company quickly complied, voluntarily recalling the following products:
- Natrelle Saline-Filled breast implants
- Natrelle Silicone-Filled breast implants
- Natrelle Inspira Silicone-Filled breast implants
- Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled breast implants
Breast implant maker Allergan also recalled several tissue expander products.
“Although the overall incidence of BIA-ALCL appears to be relatively low, once the evidence indicated that a specific manufacturer’s product appeared to be directly linked to significant patient harm, including death, the FDA took action to alert the firm to new evidence indicating a recall is warranted to protect women’s health,” said then-FDA Principal Deputy Commissioner Amy Abernethy in the press release.
The Allergan Breast Implant Cancer Class Action Lawsuit is In re: Allergan Biocell Textured Breast Implant Product Liability Litigation, Case No. BER-L-5064-20, in the Superior Court of New Jersey in and for Bergen County.
Join a Free Breast Implant Side Effects Class Action Lawsuit Investigation
If you had this issue, fill out the form on this page or follow the link below for more information.
GET HELP – IT’S FREE
Join a Free Breast Implant Side Effects Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.