Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
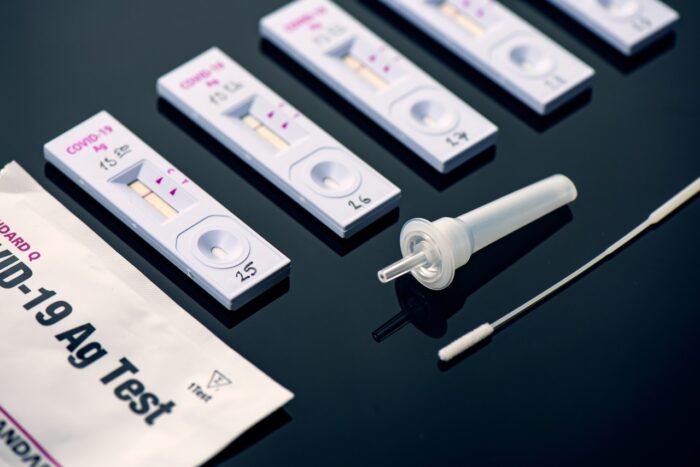
FDA LuSys Laboratories COVID-19 Antigen Test Recall:
- Who: LuSys Laboratories COVID-19 Antigen tests, sold under various names, are being recalled.
- Why: The tests are not authorized for sale in the United States, and it has not been verified that they perform accurately.
- Where: The recall is nationwide.
The U.S. Food and Drug Administration (FDA) has issued a Class 1 recall, the most serious type of recall, of a COVID-19 antigen test made by LuSys Laboratories.
The recall notice was posted to the FDA website Mar. 14 and applies to LuSys Laboratories COVID-19 Antigen Tests (Nasal/Saliva) and COVID-19 IgG/IgM Antibody Tests that may also be sold under the company names Luscient Diagnostics and Vivera Pharmaceuticals or with the trade name EagleDx.
“LuSys Laboratories is recalling these tests because they do not have an Emergency Use Authorization, 510(k) or PMA [Pre-Market Approval] and therefore cannot be legally marketed and distributed in the United States,” the FDA said.
It said LuSys Laboratories did not provide appropriate validation data to show that the tests can perform accurately. This means there is a risk of potential false negative, false positive and misinterpretation of results from these tests.
“Use of these devices may cause serious injuries or death,” the notice says.
The FDA said LuSys Laboratories has received no complaints or reports of injuries, deaths or adverse events so far.
The company is not currently facing legal action over the recall, but Top Class Actions follows recalls closely as they sometimes end in class action lawsuits.
Those who are at risk include people who were tested for SARS-CoV-2 with the product and health care providers and other organizations who used the LuSys Laboratories COVID-19 Antigen Test.
LuSys Warns Public to Immediately Stop Using Product
LuSys Laboratories sent Urgent Medical Device Recall letters to device customers, distributors and other U.S. consignees on Jan. 13 and Jan. 24, requesting they “immediately stop using” the tests.
“If the tests were distributed to third parties, immediately provide all consignees with a copy of this recall notification. Discard, destroy or return all COVID-19 tests manufactured or distributed by LuSys Laboratories, Inc,” the FDA said.
Last month, global diagnostics company SD Biosensor, Inc announced it was recalling one of its brands of at-home COVID-19 tests after discovering the tests were illegally imported into the United States.
The news comes after approximately 200,100 Flowflex SARS-COV-2 Antigen Rapid Tests (Self-Testing) were recalled by the FDA as the tests were never granted approval by the agency.
What do you think of the distribution of these tests that were not federally approved? Let us know in the comments!
Don’t Miss Out!
Check out our list of Class Action Lawsuits and Class Action Settlements you may qualify to join!
Read About More Class Action Lawsuits & Class Action Settlements:















9 thoughts onFDA Recalls Unauthorized COVID-19 Antigen Tests
Soo ….What’s going on with this currently ?? Add me
We have bought them and used them, please add me
Please add me, I have bought these and used 4 of them, never gave the accurate reading.
Please add me to the list
Add me
Use them to be tested as well
We have brought them and use them
Please add us
please add me i have these very ones thank you
Please add me