Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
The federal government has approved an at-home test for the coronavirus that can deliver results in a half hour, but experts are advising caution for those who use it.
Announced Tuesday, the Food and Drug Administration heralded the test as an important advance in mitigating the spread of COVID-19 and diagnosing those who’ve been exposed.
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” director of FDA’s Center for Devices and Radiological Health Jeff Shuren said.
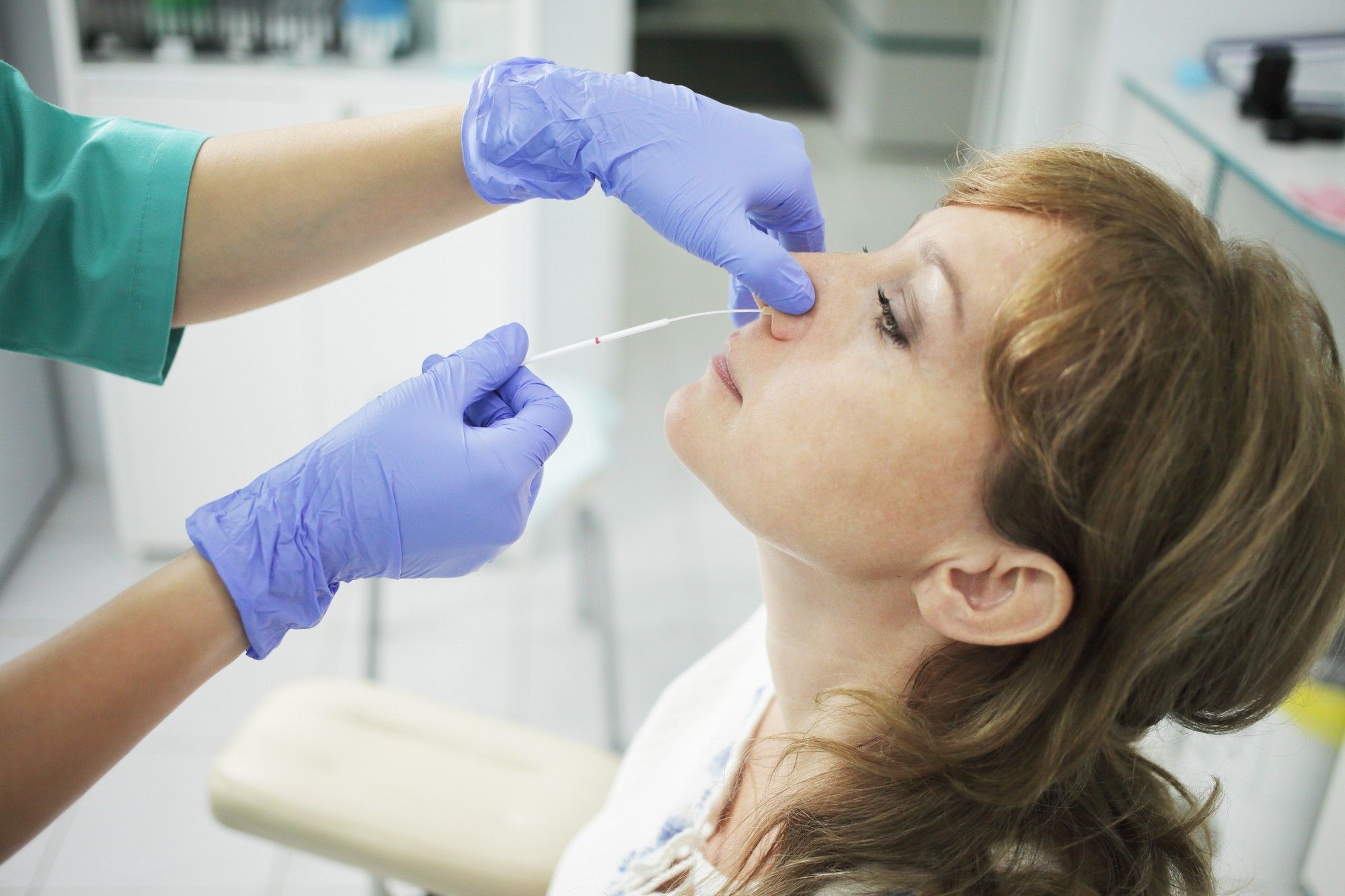
While the at-home test requires a prescription, anyone over the age of 14 can legally administer it, according to an FDA statement. The California-based medical equipment maker producing the at-home test, Lucria Health, says it will retail for under $50.
The at-home test is a battery-powered diagnostic tool made up of two nasal swabs, according to reporting by The New York Times. After the swabs are swirled in each nostril, the sample is placed into a vial of chemicals, inserted into a cartridge and read by the device. Within 30 minutes, the machine lights up with the according result.
The FDA’s approval of the at-home test marks the first of its kind created during the coronavirus pandemic, eliminating the need of a third party and can be done anywhere.
Lucria Health’s at-home test uses a method called “loop-mediated isothermal amplification reaction” or LAMPS, according to The New York Times, a similar process to the polymerase chain reaction (PCR) tests, a benchmark standard technique used during the pandemic and considered the most accurate.
Lucria Health says studies they conducted show their at-home test was able to detect 94.1% of the COVID-19 infections found by PCR tests. The company further claims the at-home test accurately identified those who are negative for the virus in 98% of the tested samples.
Omai Garner, a diagnostics expert and clinical microbiologist at the University of California, Los Angeles told The New York Times this new at-home test should be taken with caution because Lucria Health only tested and studied the at-home test on those showing symptoms.
Lucria Health has a disclaimer that the at-home test “has not been evaluated” in asymptomatic people.
“No test is perfect,” George Mason University epidemiologist Saskia Popescu told the New York Times, adding any test should not be relied on solely as an infection prevention measure and risks should continue to be weighed against benefits.
“We need more accessible and fast lab-based testing,” Popescu said.
The FDA sped up the approval of this at-home test using emergency government powers. The Emergency Use Authorization enables the FDA to lower safety standards any tests would have to clear normally.
Dr. Robin Patel of the Mayo Clinic said an at-home test option represents the future in the field of infectious diseases, but advised care in moving forward.
“I think increased testing closer to patients, including in the home, is the way of the future, but there are considerations that have to be addressed to make sure that this is done in a safe and effective way,” he told The Associated Press.
The Associated Press reports the FDA has cleared close to 300 different types of coronavirus tests. More than two dozen companies have been scrambling to get the first rapid at-home test on the market.
This will mark the second at-home test for an infectious disease the FDA has approved throughout its history, according to The Associated Press. The agency approved the OraQuick swab as an HIV diagnostic in 2012.
“We look forward to proactively working with test developers to support the availability of more at-home test options,” FDA director Jeff Shuren said.
The news of the at-home test is expected to ease the overwhelming response at testing centers across the country.
Lucria Health’s at-home test for coronavirus will be limited to those in California and Florida at first, NPR reports. The devices will be available in the rest of the U.S. by spring.
Do you anticipate using an at-home test for coronavirus when it becomes available? Let us know why or why not in the comments below.
Coronavirus Lawsuits & Legal Issues
Since the COVID pandemic shut down the country, Top Class Actions has been keeping you up to date on the latest Coronavirus lawsuits and legal issues.
Read More Lawsuit & Settlement News:
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.