Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.

The first ranitidine recall was issued on Sept. 23, 2019, by Sandoz, a maker of one of these generic forms of Zantac, and several more have followed since. The brand name version of the drug was also recalled by its manufacturer, Sanofi, in Oct. 2019.
Now, two more companies have chosen to issue a voluntary ranitidine recall over several lots of the medication.
These recalls were sparked by concerns over contamination with an impurity known as N-nitrosodimethylamine (NDMA), which is considered a probable human carcinogen, or substance capable of causing cancer in humans.
The U.S. Food and Drug Administration (FDA) issued a statement on Sept. 13, 2019, over this concern, noting that research found “low levels” of NDMA in some ranitidine medicines. While the FDA didn’t say that patients should stop taking ranitidine, the agency did note that people should be aware of this potential risk, talk to their doctor if they have any concerns, or consider using another approved medication if they use an over-the-counter version of the drug.
The most recent recalls are from Denton Pharma, which does business as Northwind Pharmaceuticals, and Appco Pharma. Denton Pharma, based in Indiana, issued a voluntary ranitidine recall of 10 lots of the drug manufactured by Glenmark after finding “unacceptable” levels of NDMA. Appco Pharma recalled 8 lots nationwide because of the risk of NDMA.
Basics of Ranitidine
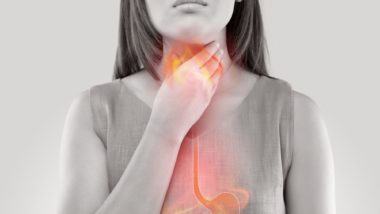
Ranitidine is an acid reduction drug, often used to treat and prevent ulcers and conditions associated with excess stomach acid.
Ranitidine has been linked with certain complications, including mild side effects like headache, constipation, or diarrhea. Unfortunately, ranitidine has also been linked with a serious contamination issue.
Ranitidine, NDMA, and Cancer Risk
The industrial waste product N-nitrosodimethylamine (NDMA) is a probable human carcinogen and has previously been discovered as a contaminant in valsartan and other ARB blocker medications. In September 2019, drugmakers began to announce a voluntary ranitidine recall due to the contamination risk of NDMA and its potential to cause cancer in patients.
Filing a Ranitidine NDMA Lawsuit
If you or someone you love has suffered from stomach cancer or bladder cancer after taking ranitidine, you may be able to file a lawsuit and pursue compensation. Of course, filing a lawsuit cannot take away the pain and suffering caused by a cancer diagnosis, nor can it bring a loved one back to life, but it can at least help to alleviate the financial burden incurred by medical expenses, lost wages, and more.
Filing a lawsuit can be a daunting prospect, especially after a cancer diagnosis, so Top Class Actions has laid the groundwork for you by connecting you with an experienced attorney. Consulting an attorney can help you determine if you have a claim, navigate the complexities of litigation, and maximize your potential compensation.
Join a Free Zantac Cancer Lawsuit Investigation
If you or a loved one was diagnosed with stomach cancer or bladder cancer after taking Zantac or another ranitidine medication, you may qualify to join this Zantac cancer lawsuit investigation. Learn more by filling out out the form on this page for a free case evaluation by a Zantac cancer injury lawyer.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Zantac Cancer Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.