Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
The Zantac ruling also provided plaintiffs with specific guidance for moving forward with the massive MDL and allowed them another chance to replead several critical claims.
As a result of hundreds of lawsuits being filed across the country by consumers who took the heartburn medication, the MDL was created on Feb. 6, 2020, transferring the many lawsuits that had been filed in federal court to the U.S. District Court for the Southern District of Florida. In three “Master Complaints” filed in June 2020, plaintiffs who took the medication raised claims under federal law and the laws of all 50 states, the District of Columbia, and Puerto Rico against several generic manufacturers and repackagers of the over-the-counter and prescription drug.
The counts in the complaints — including a personal injury complaint, a proposed consumer class, and a third-party payor proposed class action — include claims raised under consumer protection laws, strict products liability, failure to warn, negligence, deceptive trade practices, breaches of express and implied warranty, wrongful death, and others.
Notably, the consumer class action raised 314 counts on behalf of consumers throughout the nation.
As summarized in the 52-page order, the generic drug manufacturer defendants in the case relied on two Supreme Court opinions, arguing that state-law claims alleging design defect and failure to warn “are preempted because they cannot remedy design defects or provide additional warnings while remaining in compliance with federal law.” Additionally, they contended that the same rulings were also applicable to the drug’s repackagers.
Specifically, the FDA has certain requirements for making any changes to a drug or its labeling — the manufacturers would first need to seek permission from the agency to make such alterations in order to comply with state law.
However, the plaintiffs maintained that federal law could not preempt their state law claims because they concerned the misbranding of the drug at the time the product was sold — as well as the manufacturers’ failure to warn or take other measures that they could have, while still complying with federal law. They also argued that absolute liability should attach to the companies that repackaged Zantac since they profited from the marketing of the drug.
Judge Rosenberg held in the Zantac ruling that despite the plaintiffs’ misbranding argument, the claims alleging the design defect and failure to warn should be preempted, as well as the product and mislabeling claims.

According to commentary published by Law360, co-counsel for the plaintiffs acknowledged how critical the ruling is in proceeding with the litigation, stating, “These rulings are especially important in light of the 60,000-plus individuals diagnosed with cancer who have already submitted claims to the MDL registry, as well as approximately 1,000 cases filed to date in federal and state courts across the country.”
The Zantac Recall
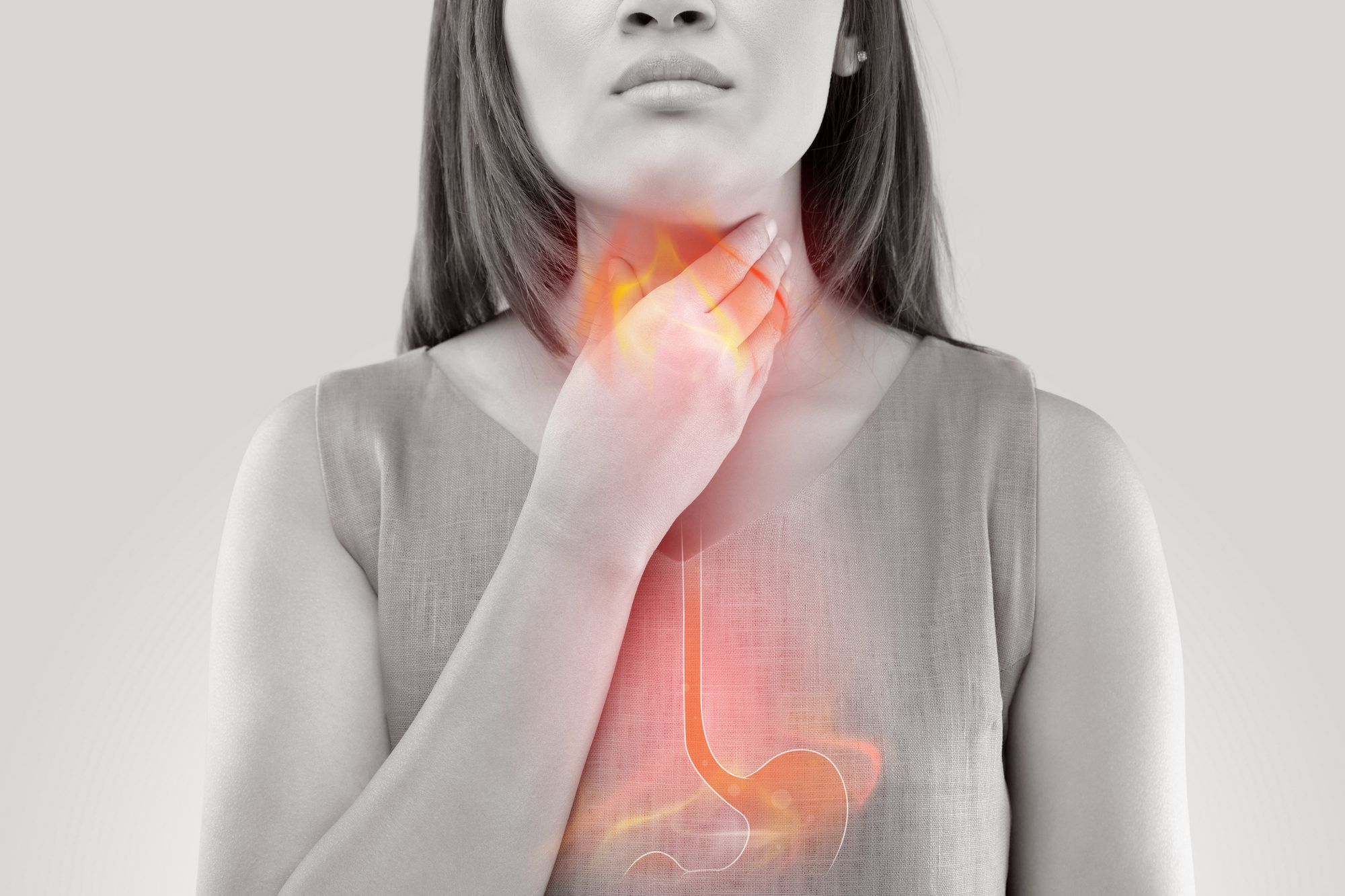
Once widely used as a medication for heartburn, acid reflux, and GERD, Zantac — the brand name for ranitidine — has been pulled off the market after studies revealed alarming levels of a carcinogen.
On April 1, 2020, the FDA requested that all drug manufacturers of ranitidine products issue voluntary recalls after testing indicated the presence of N-Nitrosodimethylamine (NDMA) — a compound that has been found to be a “probable human carcinogen” by the FDA, the Environmental Protection Agency, and the International Agency for Research on Cancer.
While the FDA has determined that the acceptable daily intake for NDMA is 96 nanograms, the agency found that the levels in ranitidine can rise when the product is stored at higher temperatures, and can increase over time.
The FDA has advised that anyone taking Zantac or a ranitidine product in any form should stop use immediately and safely discard it. Consumers should consult with their health care professionals concerning suitable alternatives that do not carry the same risks — the FDA has not found the carcinogen NDMA in several other drug options that it has tested, which are used to treat the same conditions.
The Zantac Ruling is In re: Zantac (Ranitidine) Products Liability Litigation, Case No. 9:20-md-02924, in the United States District Court for the Southern District of Florida.
Join a Free Zantac Cancer Lawsuit Investigation
If you or a loved one was diagnosed with stomach cancer or bladder cancer after taking Zantac or another ranitidine medication, you may qualify to join this Zantac cancer lawsuit investigation. Learn more by filling out the form on this page for a free case evaluation by a Zantac cancer injury lawyer.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Zantac Cancer Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.