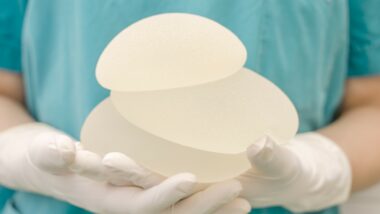
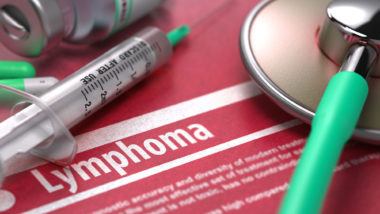
 While acknowledging a possible link between textured breast implants with cancer of the immune system, the U.S. Food and Drug Administration (FDA) announced it would continue to allow the implants to be sold in the United States.
While acknowledging a possible link between textured breast implants with cancer of the immune system, the U.S. Food and Drug Administration (FDA) announced it would continue to allow the implants to be sold in the United States.
In a May 2, 2019, statement, the FDA concluded that while there are numerous reports linking textured breast implants with cancer, the risk was not high enough to justify taking them off the U.S. market.
France has banned certain types of textured breast implants, according to The New York Times. And the International Consortium of Investigative Journalists reported that Canada and the Netherlands have suspended the sale of textured breast implants as a “precautionary measure.”
Implants with “a textured or slightly roughened surface” have been associated with anaplastic large-cell lymphoma, a rare type of non-Hodgkin lymphoma, according to the Times, and “the vast majority of the cases have occurred in women with textured implants, mainly those made by Allergan.”
The FDA said that the risk, though increased, was still low, and there is not enough data to justify banning the implants. The agency did caution that “women considering a breast implant should be aware of these risks.”
Some patient advocates were disappointed with the agency’s decision, but others were heartened by the FDA’s assurance that it would “take steps to improve the information available to women and health care professionals about the risks of breast implants,” including the risk of anaplastic large-cell lymphoma.
Jamee Cook, a patient advocate and co-founder of Breast Implant Victim Advocacy, said he hopes that future warnings will help women make informed decisions about their breast implants.
“There is information in the manufacturer’s pamphlet, but it is 40 or 60 pages long,” Cook told the Times. “Pulling it all together on a piece of paper that the patient has to actually sit here and read and check off all these boxes, and then, if she still wants those implants, power to her, that’s her decision as a patient. But we want them to have all that data readily available before making the decision.”
If future research provides a more definitive conclusion about the safety of textured breast implants, the agency may consider banning the devices.
Women getting breast augmentation surgery have a plethora of different types of breast implants to consider, including saline, structured saline, silicone, “gummy bear,” round, smooth and textured.
Textured breast implants are designed to form a “capsule” of scar tissue around the implant, which sticks to the surface, according to the American Society of Plastic Surgeons. This makes the implants less likely to shift within the breast. These implants also decrease the risk of a tight scar capsule, a breast implant side effect that can result in hard, unnatural looking breasts.
Many consumers called for FDA action after French and Canadian took action after determining the link between breast implant associated anaplastic large cell lymphoma (BIA ALCL). BIA ALCL is a rare cancer that forms within the scar tissue around the breast implant. Whereas breast cancer affects the tissue of the breast itself, BIA ALCL is primarily restricted to the scar capsule. In some cases, the cancer may spread to the nearby lymph nodes.
At least 457 women in the United States have been diagnosed with BIA ALCL, according to FDA estimates from adverse device reports. Nine of those women have reportedly died from their cancer, though BIA ALCL is fairly easy to treat when caught early.
In February 2019, the FDA announced that it was continuing to investigate the evidence linking breast implants with cancer. However, the agency’s April 2019 announcement means that it may be years before further regulation is implemented against the potentially dangerous implants.
Join a Free Breast Implants Side Effects Lawsuit Investigation
You may qualify for this breast implant investigation under the following circumstances:
- You were implanted with textured breast implants;
- You were diagnosed with Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA ALCL); and/or
- You’ve suffered from any illness you believe is related to the implants.
Fill out the form on this page for a free case evaluation by a breast implants injury attorney.
This article is not legal advice. It is presented
for informational purposes only.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Breast Implants Side Effects Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
[email protected].