 A quick internet search of breast implant illness returns hundreds of articles about a variety of illnesses and symptoms potentially associated with breast implants.
A quick internet search of breast implant illness returns hundreds of articles about a variety of illnesses and symptoms potentially associated with breast implants.
According to Harvard Health Publishing, Breast Implant Illness (BII) is a term for a “constellation of symptoms” reported by a number of women with implants.
The symptoms include things like brain fog — difficulty remembering things or concentrating — unexplained fevers, pain in muscles or joints, dry eyes and mouth, fatigue, depression, anxiety, chest pain, chills, rashes, and even hair loss.
Research has led scientists to believe that the symptoms are linked to a condition known as autoimmune syndrome induced by adjuvants (ASIA). In the case of silicone breast implants, ASIA occurs when the silicone triggers an autoimmune or foreign body response.
A 2013 investigation published in the Netherlands Journal of Medicine stated that when the implants were removed, nearly three-quarters of women reported an immediate end of the associated symptoms.
In addition to a general display of autoimmune symptoms in ASIA, there are more specific immune-related syndromes found to have a connection to the presence of silicone breast implants in some people. These include scleroderma, fibromyalgia, and chronic fatigue syndrome.
According to Dr. Josh Axe, these more specific BIIs led the U.S. Food and Drug Administration (FDA) in the early 1990s to call for a voluntary moratorium on silicone breast implants, leading to saline implants gaining popularity.
What are Scleroderma, Fibromyalgia, and Chronic Fatigue Syndrome?
Scleroderma is a chronic connective tissue disease that can be localized or systemic. The severity of the illness depends upon the type. Scleroderma causes the skin to thicken and lose its supple texture. It is associated with an increase in scar tissue. If it remains localized to a particular region, it may never affect the body’s internal organ systems.
However, the disease can and does spread to muscles and joints. A more systemic form involves the connective tissue of the heart, lungs, kidneys, gastrointestinal tract, and circulatory system.
Chronic fatigue syndrome is characterized by unrelenting exhaustion that cannot be attributed to any other medical condition. This exhaustion must persist for a minimum of 120 days to meet the criteria necessary for this diagnosis. Chronic fatigue syndrome tends to overlap with fibromyalgia whose predominant characteristic is widespread muscle and joint pain, according to the Centers for Disease Control and Prevention (CDC).
A More Serious Link
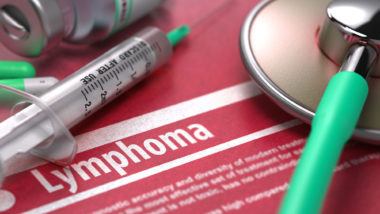
According to the U.S. Food and Drug Administration (FDA), a form of non-Hodgkin lymphoma—a cancer of the immune system—has been associated with both silicone and saline breast implants.
This cancer is known as breast implant-associated anaplastic large cell lymphoma (BIA ALCL). The association was first established in 2011, but the lack of data from longitudinal studies on breast implants has made reliable statistics difficult.
 BIA-ALCL is often found around the implant’s scar tissue capsule or in the fluid surrounding it. It is believed to be rare with agency estimates placing it as likely affecting one out of every 3,000 to 30,000 people with breast implants.
BIA-ALCL is often found around the implant’s scar tissue capsule or in the fluid surrounding it. It is believed to be rare with agency estimates placing it as likely affecting one out of every 3,000 to 30,000 people with breast implants.
In many cases, removing the implants is all that is needed in order to treat the cancer. However, sometimes the disease may spread to other parts of the body and additional treatments, including chemotherapy and radiation, may be required.
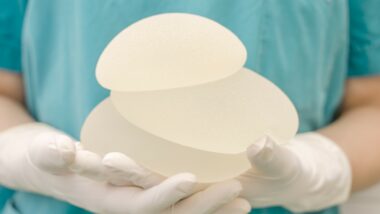
Although the exact cause of BIA-ALCL is not known, textured implants appear to pose a higher risk than smooth implants, according to Cleveland Clinic. Textured implants have a rough surface that promotes the growth of scar tissue around them, and may result in the implants staying in place longer rather than drifting across the chest or into the armpit. However, these implants may also trigger immune reactions or inflammation, which could result in the development of cancer.
Breast Implant Recalls
In July 2019, Allergan recalled several lots of its textured breast implants and tissue expanders due to concerns that the products may be linked to an increased risk of BIA ALCL.
There have been a total of 573 cases of BIA-ALCL reported worldwide. Of this number, 481 people reportedly had Allergan implants at the time of diagnosis. Twelve patients with Allergan breast implants died from the cancer.
The following Allergan Natrelle BIOCELL textured products were included in the voluntary recall:
- Allergan Natrelle Saline-Filled Textured Breast Implants
- Allergan Natrelle Silicone-Filled Textured Breast Implants
- Allergan Natrelle 410 Highly Cohesive Anatomically Shaped Silicone- Filled Textured Breast Implants
- Allergan Natrelle 133 Plus Tissue Expander
- Allergan Natrelle 133 Tissue Expander with Suture Tabs
Although Allergan’s tissue expanders have not been associated with BIA-ALCL, the company recalled the products since they use the same BIOCELL texture as the recalled breast implants. Tissue expanders are only indicated to be used for six months, but the FDA is not sure what duration of exposure may lead to BIA-ALCL
The FDA issued a Class I recall, its most serious type. According to the agency, “use of these devices may cause serious injuries or death.”
Because the risk of developing BIA-ALCL is so low, the FDA does not recommend that those with Allergan breast implants have them removed if they are not experiencing any symptoms of the disease. Anyone with questions or concerns about their recalled Allergan breast implants should speak with their doctor.
Although Allergan has offered to cover the cost of new Allergan implants for people who wish to have their recalled implants removed, the company will not pay for the surgery to remove the old implants. This has left many women with the recalled implants in a conundrum: keep the implants that may increase the likelihood of developing cancer or breast implant illness, or pay to have them removed. It costs about $3,000 to have implants removed, and between $6,000 and $12,000 for replacement surgery. As many insurance companies will not pay for these procedures, women are forced to choose between their finances and their health.
For women who opted for implants after undergoing a double mastectomy due to breast cancer, the decision may be especially difficult.
“The whole point of a double mastectomy was peace of mind from cancer, so to find the implants used in reconstruction pose their own cancer risk has been very upsetting,” said a woman who spoke to Insider. “It makes me question whether I should have gotten implants in the first place.”
Join a Free Breast Implants Side Effects Lawsuit Investigation
You may qualify for this breast implant investigation under the following circumstances:
- You were implanted with textured breast implants;
- You were diagnosed with Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA ALCL); and/or
- You’ve suffered from any illness you believe is related to the implants.
Fill out the form on this page for a free case evaluation by a breast implants injury attorney.
This article is not legal advice. It is presented
for informational purposes only.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Breast Implants Side Effects Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
[email protected].









One thought on Is Breast Implant Illness Real?