Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
Individuals with textured breast implants may want to be on the lookout for symptoms of BIA ALCL.
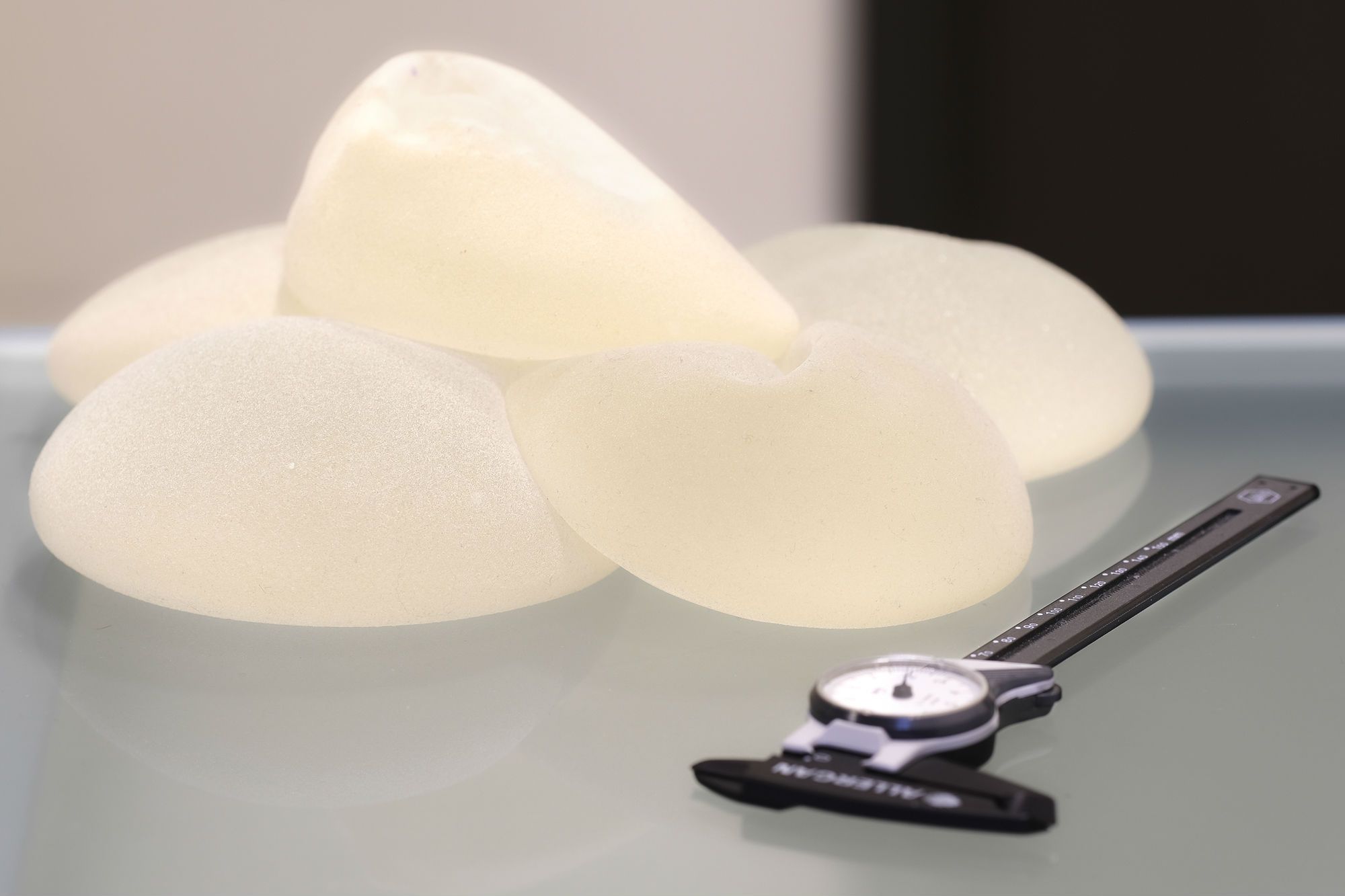
How Are Textured Breast Implants Different?
Unlike other breast implants, textured breast implants encourage the growth of scar tissue around the implant. Some doctors prefer these implants because they are supposed to reduce the risk of the implants shifting and the body forming hard scar tissue. The goal of textured implants is to prevent hard, unnatural looking breasts after breast augmentation surgery.
Who Manufactures Textured Breast Implants?
Most textured implants (also known as “cohesive” implants) are made by Allergan, a global pharmaceutical firm headquartered in Dublin. The product line is called BIOCELL. The company recalled all of these textured breast implants in July 2014.
Other companies that manufacture and market textured implants are Johnson & Johnson subsidiaries Mentor and Sientra. However, out of 13 deaths attributed to textured or cohesive breast implants, 12 were found to have Allergan implants. Mentor and Sientra products have not been recalled as textured breast implants represent only 5 percent of Mentor sales, while Sientra’s are no more than 20 percent, according to MedTechDive.com.
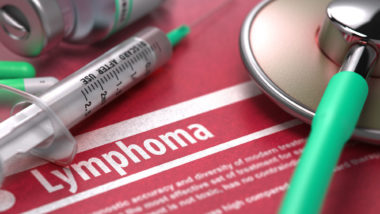
What Is BIA ALCL?
Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA ALCL) is a sub-type of T-cell Non-Hodgkin’s Lymphoma that develops in the scar tissue capsule around the breast implant. This cancer differs from regular breast cancer because it does not affect breast tissue. Instead, the cancer is usually confined to the scar tissue capsule.
What Are the Symptoms of BIA ALCL?
According to the Association of Breast Surgery, symptoms of BIA ALCL include scar capsule thickening leading to a hardening breast, a mass, and swelling. Other symptoms may include breast pain, changes in breast appearances, fluid buildup, and a skin rash.
How Is BIA ALCL treated?
Luckily, when the condition is caught early, BIA ALCL is usually easy to treat. In the early stages, the cancer will not have metastasized to the lymph nodes or other parts of the body, meaning that it stays confined in the scar tissue capsule around the implant.
If the cancer only affects the area surrounding the implant, treatment usually involves removing the breast implant and scar capsule around the implant. If the cancer has spread, treatment may involve the removal of these tumors. In advanced cases, chemotherapy, radiation therapy, and stem cell transplant therapy may be necessary to resolve the disease.
How Common Is BIA ALCL?
In July, the U.S. Food and Drug Administration (FDA) reported that at least 573 women have developed BIA ALCL since 2011, when the condition was first warned of by the agency. Of these women, 33 died as a result of cancer linked to breast implants.
What Does the FDA Say About BIA ALCL?
In February, the FDA warned that textured breast implants may be linked to BIA ALCL. Due to the number of women who have developed BIA ALCL since 2011, the FDA is reportedly working with the American Society of Plastic Surgeons to understand the risk.
“Though the number of identified cases of BIA-ALCL is small compared to the estimated 1.5 million patients who receive breast implants worldwide every year, confirmed data and published information reviewed to date suggests that patients with breast implants have an increased risk of BIA-ALCL,” the FDA said.
Other foreign regulatory agencies have taken action against textured breast implant manufacturers. In April 2018, France banned certain textured breast implants due to concerns about BIA ALCL. Health Canada is considering following in France’s footsteps by banning some textured breast implants.
In comparison to other countries around the world, the FDA has taken a more modest approach to handling the possible BIA ALCL risks that may be associated with textured breast implants. This response reportedly leaves critics, activists, and some patients wondering if more action may be needed to fully inform the public of the risks and symptoms of BIA ALCL.
The FDA has requested that the makers of textured breast implants share information about the products’ possible risks with medical professionals. The FDA recommends that this information be shared in the form of pamphlets given to doctors, who can then pass on the information to patients. This recommendation was made after the FDA acknowledged that textured breast implants may be more often linked to BIA ALCL than other kinds of breast implants.
However, this recommendation is just that — a recommendation, as opposed to a requirement.
Currently, breast implant makers are not required to provide this information, and doctors are not required to pass this information along to patients. This means that many patients may be in the dark about all of the possible risks that may be associated with the medical products. Some manufacturers may choose to put profits over public awareness by not telling doctors about the possible risks.
This relatively passive approach to the BIA ALCL risk has garnered criticism from activists. The New York Times reports that around 70,000 people have signed a petition asking the FDA to require manufacturers to inform both doctors and patients of the possible risks of textured breast implants by providing “risk checklists” covering all dangers, along with labeling the products themselves.
If I Have a BIOCELL Implant, Should I Have it Removed?
Unless you are experiencing symptoms such as those described above, the FDA is not recommending that you have your textured breast implants removed. If you do have symptoms, you should speak with your physician and ask for further tests and evaluation. A diagnosis of BIA-ALCL requires a thorough physical examination, including medical imaging and an assessment of fluid and/or tissues surrounding the implant. If a BIA-ALCL diagnosis is confirmed, the implant and surrounding scar tissue should be surgically removed.
Keep in mind that in the majority of cases, BIA-ALCL symptoms manifest many months or even years after implantation.
What About Taking Legal Action?
In the case of injury from any medical product or device, legal representation may be pursued. If any medical device that has been implanted in your body has caused harm, you’ll need a record of the manufacturer, the serial number or other identifier, and the name of the implant. This information should be included with your medical records at the clinic where the operation was carried out.
Join a Free Breast Implants Side Effects Lawsuit Investigation
You may qualify for this breast implant investigation under the following circumstances:
- You were implanted with textured breast implants;
- You were diagnosed with Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA ALCL); and/or
- You’ve suffered from any illness you believe is related to the implants.
Fill out the form on this page for a free case evaluation by a breast implants injury attorney.
This article is not legal advice. It is presented
for informational purposes only.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Breast Implants Side Effects Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.