Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
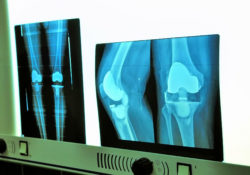
Aseptic tibial loosening, or loosening that happens without an infection, is a side effect of bone cement failure. Patients complaining of persistent pain, newly evident chronic pain, instability, swelling, or a decreased range of motion might be experiencing aseptic tibial loosening.
Not all knee replacement systems require cement. Some types of knee replacement systems rely on the bone growing in and around the implant. In other types of implants, bone cement anchors the implant components to the tibia and the femur.
Low, medium or high-viscosity cement may be used during surgery to stabilize the implant components. High-viscosity cement is thick and easier for the surgeon to control, which often makes it the preferred choice. The high-viscosity cement mixes quicker and sets in place faster because it contains less liquid.
Unfortunately, some doctors and researchers have linked the high-viscosity formulas with poor bonding. Among the bone cement products under investigation for failing to bond or for premature breakage of a bond are:
- Cobalt HV Bone Cement (Biomet/DJO Surgical)
- DePuy CMW 1 Bone Cement
- Simplex HV Bone Cement (Stryker/Howmedica)
- SmartSet HV Bone Cement (DePuy)
DePuy, maker of the two of the bone cements on the list, is a subsidiary of Johnson & Johnson.
Failed Bone Cement and Knee Revision Surgery
In 2016, the National Center for Biotechnology Information (BNBI) analyzed 13 incidents involving the knee implant failing to keep attached to the cement or the implant loosening from the bone at the front of the tibia. The problem was determined to be high viscosity cement; in implants that used low or medium viscosity bone cement, there were no similar debonding issues.
One woman filed a lawsuit alleging DePuy CMW 1 Gentamicin bone cement was at fault for the failure of her left knee’s implant. She underwent surgery to have the knee replaced in January 2009, but by late 2011, she said her knee was aching and throbbing.
Even though an X-ray showed the knee replacement system was in a good position, a bone scan revealed loosening of the tibial component. As a result, she underwent a total knee revision surgery in May 2012.
According to her complaint, her doctor said the tibial component of the original knee replacement surgery had become loose from the bone because the bone cement failed to properly adhere to the component’s surface.
DePuy CMW 1 Gentamicin bone cement was approved through the FDA’s fast-track program that allows products that are substantially equivalent to those already approved by the FDA to skip some clinical testing requirements. DePuy submitted CMW 1 Gentamicin bone cement for approval on Oct. 24, 2005, and the FDA approved it on Nov. 22, 2005.
A manufacturing change from micronized particles to non-micronized particles purportedly led to larger particulate matter in the CMW1 Gentamicin bone cement, which allegedly hindered the cement’s adherence properties. The lawsuit alleges DePuy failed to adequately test the results of the manufacturing changes before placing the bone cement on the market.
If you or a loved one underwent revision knee replacement surgery or your doctor is recommending revision surgery three years or less after the initial implant and a bone cement was used, you may qualify to file a knee replacement revision surgery lawsuit. See if you qualify by filling out the free form on this page.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Knee Replacement Cement Failure Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
Oops! We could not locate your form.












