Update:
- Exela Pharma Sciences added 14 more lots to an ongoing voluntary recall of 49 lots of its Sodium Bicarbonate Injection, USP, 8.4%, 50 mEq/50 mL vials, bringing the total number recalled to 63 lots.
- The pharmaceutical company distributed the newly recalled sodium bicarbonate injections lots between Oct. 26, 2021, and April 25, 2022.
- Exela initiated the recall after becoming concerned that the glass vials housing the sodium bicarbonate injections could break and shatter when being prepared under pressure for administration.
- The company says it has received five reports of flying glass causing injury to skin, eyes and/or other body parts.
- Exela says there have been no reports of vial breakage or injury related to the 14 lots now added to the recall.
Sodium Bicarbonate recall overview:
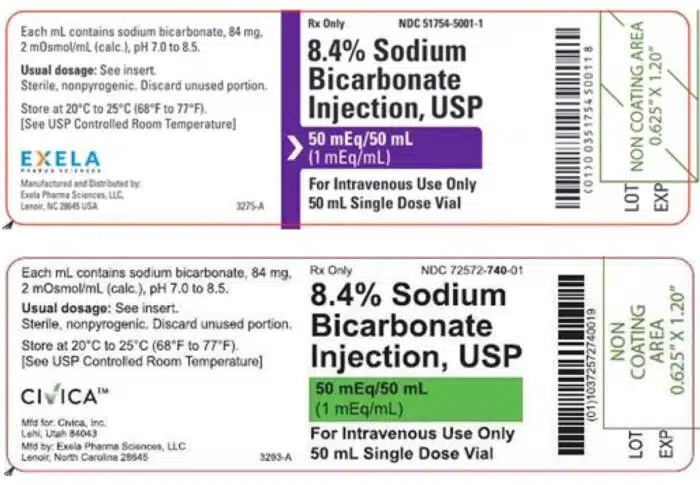
- Who: Exela Pharma Sciences has voluntarily recalled 49 lots of its Sodium Bicarbonate Injection, USP, 8.4%, 50 mEq/50 mL vial, 20-count cartons.
- Why: The recall was initiated over concern the vials can shatter and spray glass when under pressure.
- Where: The recall is nationwide.
(Oct. 17, 2022)
Exela Pharma Sciences has voluntarily recalled 49 lots of Sodium Bicarbonate Injection, USP, 8.4%, 50 mEq/50 mL vial, 20-count cartons at the consumer level over concerns the vials can break when pressurized.
The recall was initiated after Exela discovered that the Sodium Bicarbonate Injection vials can shatter and spray glass while being put under pressure during administration of the product, according to a recall published Oct. 13 by the U.S. Food and Drug Administration (FDA).
Exela says it has received five reports of injuries occurring as a result of flying glass from shattered vials, including to the skin, eyes and other body parts.
The Sodium Bicarbonate Injection product is used to treat metabolic acidosis and labeled with Exela brand and Civica brand have expiration dates between October 2023 and March 2024, according to the recall.
Exela does not expect recall to lead to product shortage
Exela says that the recall is not expected to result in a shortage of the Sodium Bicarbonate Injection product distributed nationwide to wholesalers, distributors, and customers between Dec. 16, 2021, and Aug. 10, 2022.
Customers who are affected by the recall are being notified by Exela by both email and certified mail with the company helping facilitate the return and replacement of the product, according to the sodium bicarbonate recall.
Individuals who have more questions about the recall can contact Exela directly by email or by phone at 828-341-6118, extension 1017.
Sodium bicarbonate was also involved in a recent class action lawsuit.
A consumer filed the lawsuit against Conagra Brands in September, arguing the company falsely labels that its Log Cabin pancake mixes are ‘all natural’ despite containing sodium bicarbonate.
Are you affected by the Exela sodium bicarbonate recall? Let us know in the comments!
Don’t Miss Out!
Check out our list of Class Action Lawsuits and Class Action Settlements you may qualify to join!
Read About More Class Action Lawsuits & Class Action Settlements:














