Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
Daptomycin, vancomycin recalls overview:
- Who: The U.S. Food and Drug Administration published a pair of voluntary nationwide recalls made separately by Hospira Inc. and Accord Healthcare.
- Why: The voluntary recalls are for a single lot of a Hospira vancomycin injection product and a single lot of Accord daptomycin injection products.
- Where: The recalls affect patients nationwide.
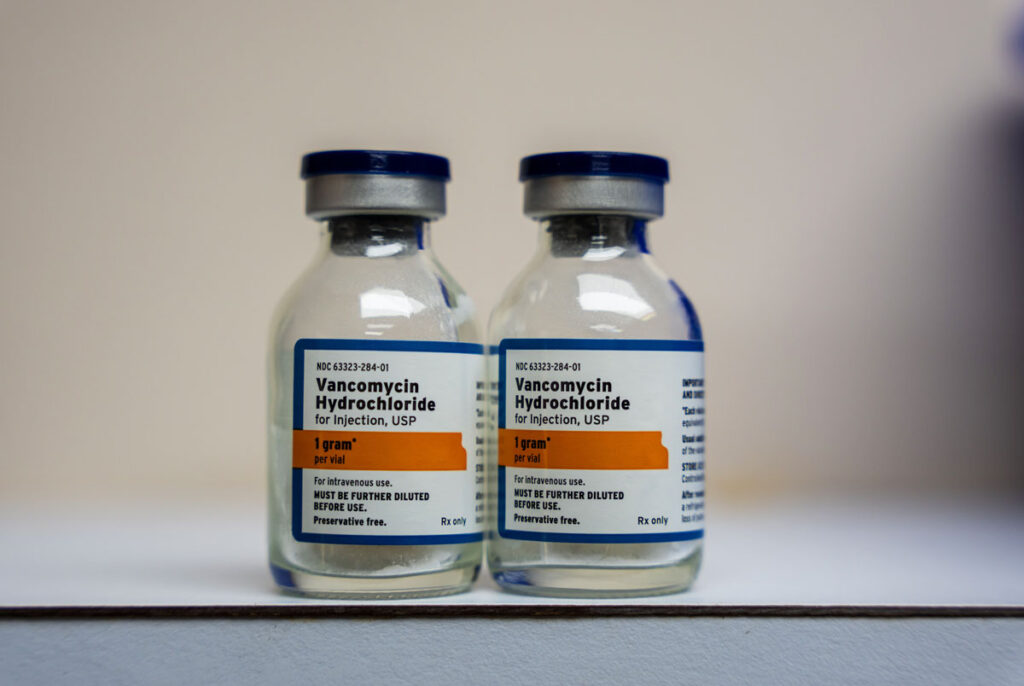
The U.S. Food and Drug Administration (FDA) published two voluntary nationwide recalls this week for vancomycin and daptomycin injection products offered by Hospira Inc. and Accord Healthcare, respectively.
Hospira, a Pfizer company, initiated a recall to the user level on Dec. 22 for one lot of its Vancomycin Hydrochloride Injection, USP 1.5g/vial Single Dose Fliptop Vial drug after discovering a single vial contained two visible glass particulates.
Wholesalers, hospitals, institutions and doctors who have an existing inventory of the recalled vancomycin hydrochloride injection lot should stop using and/or distributing it and quarantine the drug immediately, according to the vancomycin recall.
Hospira says it has not received any reports of adverse effects that would be connected to the recall; however, the company warns patients who had the recalled drug administered intravenously “may experience adverse events.”
Individuals who want more information about the recall can contact Sedgwick Inc. directly by phone at 1-800-805-3093, while healthcare providers requesting more information should contact Pfizer.
Anyone who experiences adverse reactions from the recalled vancomycin hydrochloride injection drug can file a report with the FDA’s MedWatch Adverse Event Reporting program online or by mail or fax, according to the vancomycin recall.
Accord Healthcare voluntarily recalls single lot of daptomycin
Accord, meanwhile, announced on Dec. 23 that it voluntarily recalled to the user level one lot of its Daptomycin for Injection 500 mg/vial and Daptomycin for Injection 350 mg/vial drug due to a mixup involving the products.
Accord initiated the recall after it received a product complaint from a hospital pharmacy that discovered the vials for the recalled Daptomycin for Injection 500 mg/vial were in cartons labeled as Daptomycin for Injection 350/mg vial, according to the daptomycin recall notice.
Accord says it has notified or is in the process of notifying wholesalers and distributors about the recall by letter and arranging for them to return all of the recalled products.
Wholesalers and distributors that currently have the recalled Daptomycin for Injection products should stop distributing them, the daptomycin recall states.
Individuals who have more questions about the recall can contact Accord directly by phone at 1-855-869-1081, by fax at 1-817-868-5362 or by email.
Anyone who experiences adverse reactions from the recalled Daptomycin for Injection products can file a report with the FDA’s MedWatch Adverse Event Reporting program online or by mail or fax, according to the daptomycin recall.
In other drug recall news, earlier this month, Exela Pharma Sciences added 14 lots of its Sodium Bicarbonate Injection, USP, 8.4%, 50 mEq/50 mL vials to an ongoing recall initiated over concerns the glass vials holding the injections could shatter and break when under pressure.
Are you affected by the Accord Healthcare and Hospira recalls? Let us know in the comments!
Don’t Miss Out!
Check out our list of Class Action Lawsuits and Class Action Settlements you may qualify to join!
Read About More Class Action Lawsuits & Class Action Settlements:














5 thoughts onDaptomycin, vancomycin hydrochloride injections recalled nationwide
I had total knee replacement. I ended up with a bad infection the doctor put me on an I/V of Vancomysin for 6 weeks at home . I told the doctor that the medication was making me sick. After the 6 weeks he wanted to continue it for 1 more week. I now have a severe case ot Tinnitus. Please add me.
Was overdosed of vancomycin in october 2021. Sent me into kidney failure
I have vancomycin in hospital before I had iv spot turn red itchy just uncomfortable not good feeling
My husband had a surgery in Dec 2022 and had a reaction to the vancomycin hydrochloride. His surgery was almost postponed because of a reaction he had from the IV with pre-op. He had been given the drug in previous surgeries with no side effects. Please add me.
I had C-diff in the Hospital and was given Vancomycin Hydrochl orizide and I had Sepsis Shock and was given Daptomycin through an IV…Add me