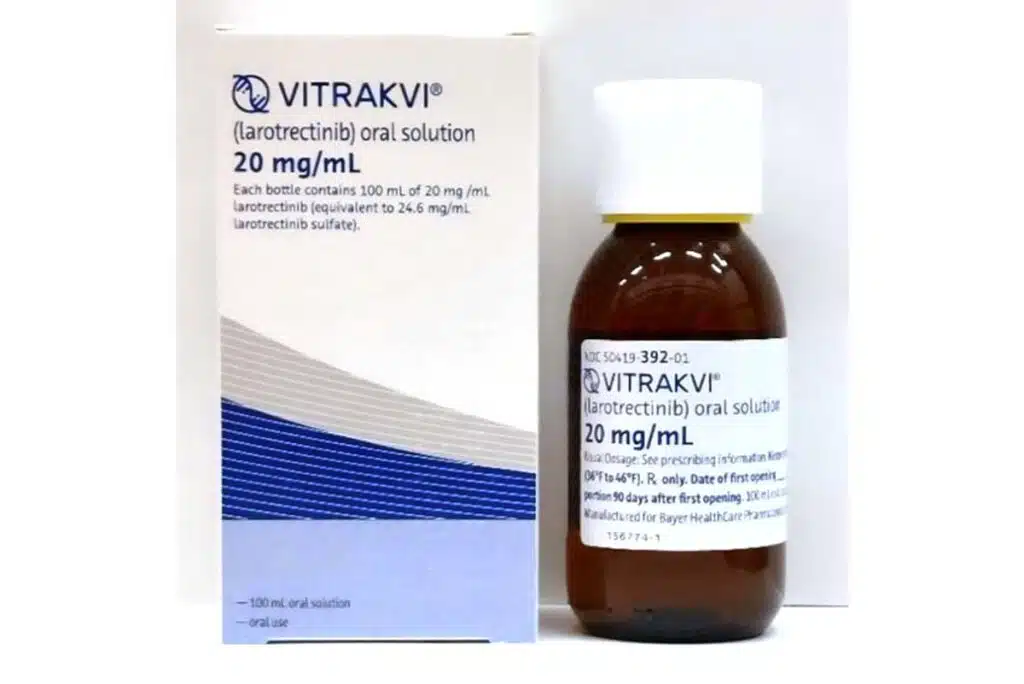
Vitrakvi recall overview:
- Who: Bayer recalled one lot of its Vitrakvi oral solution 20 milligrams per milliliter in 100 milliliter bottles.
- Why: The oral solution could be contaminated with penicillium brevicompactum, which could lead to invasive disease, especially in those who are immunosuppressed, the Bayer recall says.
- Where: The Vitrakvi recall is valid across the United States.
Bayer initiated a recall for one lot of its Vitrakvi oral solution 20 milligrams per milliliter in 100 milliliter bottles due to possible contamination with penicillium brevicompactum.
Random testing discovered the potential contamination, and, while there is little data on the impact of penicillium brevicompactum on humans, other penicillium have led to cases of invasive disease, especially in those who are immunosuppressed, the Bayer recall says.
The Bayer recall includes 100-milliliter bottles of Vitrakvi with NDC 50419-392-01, lot no. 2114228 and an expiration date of Feb. 29, 2024. Bayer distributed the impacted lot to wholesale distributors and specialty pharmacies nationwide between Jan. 3, 2023, and Feb. 13, 2023.
Stop using impacted Vitrakvi immediately, Bayer recall says
Bayer says it has not received any reports of illness related to the recall. The company is not facing legal action over the recall, but Top Class Actions follows recalls closely as they sometimes lead to class action lawsuits.
Consumers should stop using the recalled Vitrakvi immediately and can contact Qualanex at [email protected] or (888) 280-2043 from 7 a.m. to 4 p.m. CT Monday to Friday.
Customers can also reach the Bayer Medical Information Call Center at (888) 842-2937 from 8:30 am. to 6 p.m. ET Monday to Friday.
In other Bayer news, Bayer Corporation faced a class action lawsuit earlier this year, claiming it falsely advertises its One a Day Natural Fruit Bites multivitamin products as “natural” even though they contain synthetic ingredients.
Are you affected by the Vitrakvi recall? Let us know in the comments.
Don’t Miss Out!
Check out our list of Class Action Lawsuits and Class Action Settlements you may qualify to join!
Read About More Class Action Lawsuits & Class Action Settlements:
- Recall initiated for KinderMed pain, fever products due to acetaminophen instability
- Recall issued for several injection products due to presence of particulate matter
- Harris Teeter class action claims retailer sells phenylephrine decongestants that don’t work
- Botanical-Be issues recall for supplements due to diclofenac presence















18 thoughts onBayer issues recall for Vitrakvi oral solution due to microbial contamination
Please add me
please add me
please add me
please add me
Add me
Add me
also add me, thanks
add me also