Injection recall overview:
- Who: Exela recalled several lots of sodium bicarbonate injection under the Exela and Civica brands, as well as Midazolam and ELCYS cysteine hydrochloride injections.
- Why: During routine inspection, the injections were found to have silicone particulate matter.
- Where: The injection recalls affect consumers nationwide.
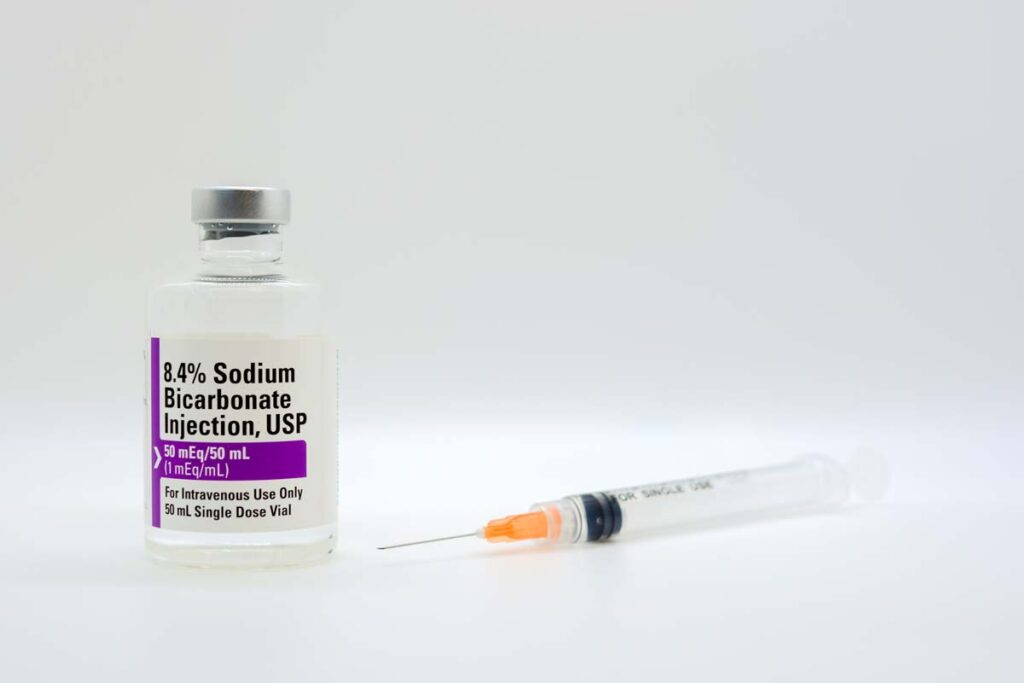
Exela issued a recall for several lots of sodium bicarbonate injection under the Exela and Civica brands, as well as Midazolam and ELCYS cysteine hydrochloride injections, due to silicone particulate matter found during routine inspection.
The recall includes the 50 milliliter single dose vial of 8.4% sodium bicarbonate Injection, 100 milliliter single dose vial of Midazolam in 0.8% sodium chloride injection and 10 milliliter single dose vial of ELCYS cysteine hydrochloride injection.
The recalled lots of sodium bicarbonate include Exela P0001429, P0001900, P0001902, P0001903, P0001909, P0001945, P0002002 and P0002052 and Civica P0001912 distributed to wholesalers, distributors and health care companies between Jan. 18, 2022, and Feb. 15, 2023.
The Midazolam recall includes lot no. 10001088 distributed from July 14, 2023, to Sept. 26, 2023, while the ELCYS is lot no. 10000798 distributed from July 20, 2023, to Aug. 1, 2023.
No reports of illness, injury related to injection recalls, Exela says
Exela says it has not received reports of illness or injury related to the recall. The company is not facing legal action over the recall, but Top Class Actions follows recalls closely as they sometimes lead to class action lawsuits.
Injecting solutions containing particulate matter may result in local irritation or swelling, according to the injection recalls. Additionally, if the particulate matter reaches the blood vessels, it can travel to various organs and block blood vessels in the heart, lungs or brain, which can cause stroke and even lead to death.
Consumers can reach Exela from 9 a.m. to 5 p.m. ET Monday to Friday at (828) 341-6118 or recall@exela.us.
In a similar recall, earlier this month, Hospira Inc. recalled one lot of its sodium bicarbonate injection and two lots of its lidocaine HCI injection due to the potential presence of glass particulate matter in the solutions.
Are you affected by the injection recalls? Let us know in the comments.
Don’t Miss Out!
Check out our list of Class Action Lawsuits and Class Action Settlements you may qualify to join!
Read About More Class Action Lawsuits & Class Action Settlements:















6 thoughts onRecall issued for several injection products due to presence of particulate matter
Add me I received 6 in total but I would like more information. Thank you
Please contact me.
I would like more information about the recalled lidocaine HCI injections, I had 8 in my back on June 12 and have had and have to this day many issues from the injections. I do not go to that doctor anymore because of this and I had to resign from my job because of the conditions!
Every month I receive injections in head, neck, and back, for pain relief. In 2018 I had a very bad reaction to the injection, which has now lead to heart trouble. Makes me wonder if I qualify for this suit. Please let me know. Thank you.
add me thanks
add me please received 3 injections total in the pass