Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
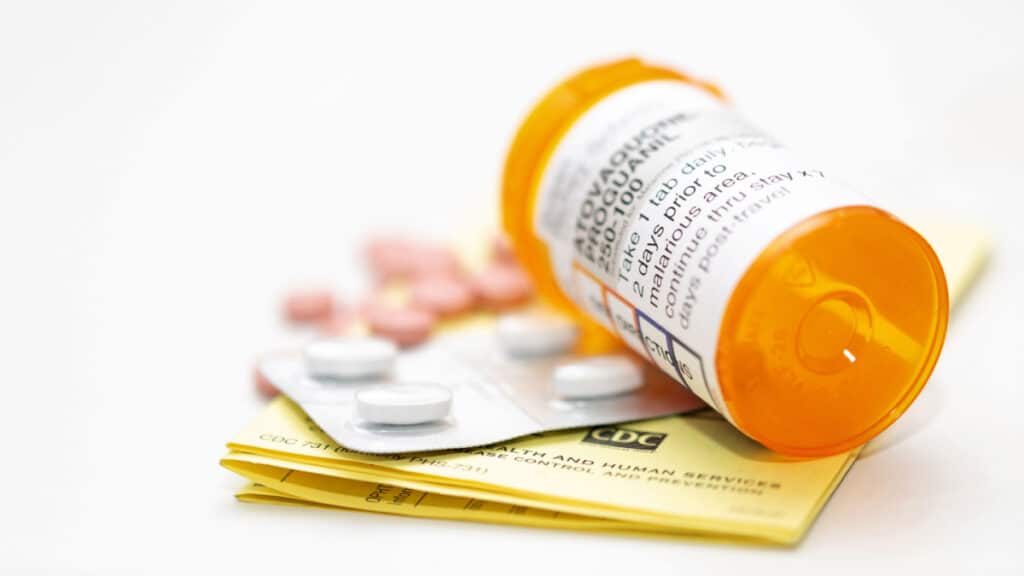
Pneumonia medication recall overview:
- Who: Camber Pharmaceuticals Inc. is recalling some of its Atovaquone oral suspension pneumonia medication.
- Why: The pneumonia medication recall is due to some of the medication having a potential Bacillus cereus contamination.
- Where: The recall is effective in the United States.
Camber Pharmaceuticals Inc. is recalling some of its Atovaquone oral suspension pneumonia medication due to a potential Bacillus cereus contamination.
The medication is for the treatment of pneumocystis jiroveci pneumonia (PCP) for those adults and children 13 and older who cannot take other infection medications, such as trimethoprim-sulfamethoxazole.
The immunocompromised population is the most at risk due to the contamination with a reasonable probability that the microbial contamination could lead to life-threatening infections such as endocarditis and necrotizing soft tissue infections, according to the pneumonia medication recall.
The pneumonia medication recall, published by the United States Food and Drug Administration (FDA), is for the lot marked E220182 with a UPC of 331722629218 and an expiration date of 12/2023. The medication was distributed to wholesalers and pharmacies nationwide. The medication comes in 210 ML containers and is identified with the National Drug Code of 31722-629-21.
Consumers should immediately stop using the medication and return or dispose of it, pneumonia medication recall says
Camber Pharmaceuticals says it has not received any reports of illness related to the recall so far. The company is not currently facing legal action over the recall, but Top Class Actions follows recalls closely as they sometimes lead to class action lawsuits.
Third-party Inmar will be notifying distributors, pharmacies and customers with mailings and emails on returning the medication.
Consumers can contact Inmar at 877-597-0878 or via email at rxrecalls@inmar.com between 9 a.m. and 5 p.m. Eastern, Monday through Friday. Consumers should contact their physician or health care provider if they have had any issues related to taking the medication.
Issues with the medication can also be reported to the FDA’s MedWatch Adverse Event Reporting program through its online form, by calling 800-332-1088 to request a form, downloading and mailing the form here or by faxing the form to 800-FDA-0178.
The FDA recently issued another recall notice for Ascent Laboratories’ anticoagulant dabigatran etexilate for having elevated levels of nitrosamine, which is known to cause cancer for those who are exposed to high levels of the drug over an extended period of time.
Are you affected by the pneumonia medication recall? Let us know in the comments.
Don’t Miss Out!
Check out our list of Class Action Lawsuits and Class Action Settlements you may qualify to join!
Read About More Class Action Lawsuits & Class Action Settlements:














2 thoughts onPneumonia medication recall announced due to possible bacterial contamination
Add me
add me please