Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
A Connecticut woman recently filed an Elmiron lawsuit against Teva Pharmaceuticals and Janssen Pharmaceuticals, alleging that the companies’ bladder medication Elmiron caused her loss of vision.
The plaintiff, Kimberly P., began taking Elmiron at the direction of her doctor back in 2005 to treat her interstitial cystitis, a medical problem of the bladder. But in about 2015, Kimberly began experiencing issues with her vision. Over the course of the next four years, Kimberly had her eyesight evaluated a number of times, and was ultimately diagnosed with permanent retinal injury and vision loss in 2019, which she alleges was caused by Elmiron toxicity.
According to Kimberly’s Elmiron lawsuit, the bladder medication did not have the proper warnings about the medication’s risks, though the manufacturers either knew or should have known about the risk of vision problems linked with the drug.
The lawsuit notes that Elmiron is designed as a long-term drug, and patients are expected to use it for years or even decades. Long-term use of a drug increases the risk of toxicity-related side effects, and, the lawsuit notes, “the retina is especially susceptible to the effects of systemic drugs…the retina has minimal ability to regenerate and is therefore at high risk of drug toxicity.”
Indeed, according to the lawsuit, both patients and health care providers had reported Elmiron’s issues, but the manufacturers failed to act on these reports by conducting further testing, withdrawing the drug from the market, or updating warning labels. Instead, the companies continued to market Elmiron as a “safe and effective prescription drug for interstitial cystitis.”
Kimberly alleges that as a direct result of using Elmiron, she suffered from both physical and emotional injuries—primarily, loss of vision and diagnosis of retinal macula dystrophy.
What is Elmiron?
Elmiron (pentosyn polysulfate sodium) is an oral medication approved by the U.S. Food and Drug Administration (FDA) back in 1996 as a treatment for interstitial cystitis, along with painful bladder symptoms. Essentially, interstitial cystitis is a condition involving pain and/or pressure in the bladder area without a clear cause.
Elmiron is, so far, the only medication approved by the FDA for this condition. Elmiron is believed to work by forming a layer on the bladder wall to protect against harmful irritants, but as the cause of interstitial cystitis is still unknown, this is still a theory.
Teva Pharmaceuticals developed Elmiron and licensed the drug to Janssen Pharmaceuticals (parent company of Johnson & Johnson), which is now the manufacturer and distributor of Elmiron.
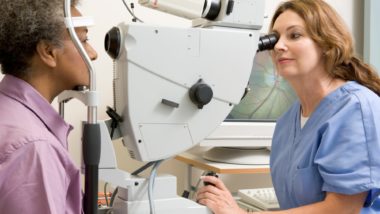
 Elmiron Vision Problems
Elmiron Vision Problems
A few studies in recent years have linked long-term use of Elmiron with vision problems, including retinal or macular degeneration. Also known as maculopathy, macular degeneration is a disease that affects the macula, the central portion of the retina. The macula is responsible for several key elements of vision: central vision, the majority of color vision, and fine detail.
Filing an Elmiron Lawsuit
If you or someone you love has suffered from Elmiron side effects like vision loss or retinal macula dystrophy, you may be able to file a lawsuit and pursue compensation. Of course, filing a lawsuit cannot take away the pain and suffering caused by vision problems, but it can at least help to alleviate the financial burden incurred by medical expenses, lost wages, and more.
Filing a lawsuit can be a daunting prospect, especially while dealing with medical issues, so Top Class Actions has laid the groundwork for you by connecting you with an experienced attorney. Consulting an attorney can help you determine if you have a claim, navigate the complexities of litigation, and maximize your potential compensation.
Kimberly’s Elmiron Lawsuit is Case No. 3:20-cv-00406, in the U.S. District Court for the District of Connecticut.
If you were treated with Elmiron and developed vision problems, you may be eligible to participate in this free Elmiron side effects lawsuit investigation. Fill out the form on this page for more information.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Elmiron Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.
Oops! We could not locate your form.

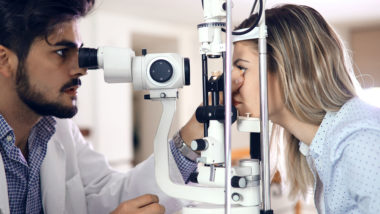
 Elmiron Vision Problems
Elmiron Vision Problems