Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.

The FDA is requiring that Noroxin and other fluoroquinolone antibiotics’ warning labels be updated to include this information on their warning labels and patient medication guides, to help prevent potential incidents.
In addition, the FDA stated fluoroquinolones should not be prescribed to high risk patients such as those with peripheral atherosclerotic vascular diseases, hypertension, or certain genetic disorders, or who are elderly.
The FDA issued this announcement after reviewing case reports from the FDA Adverse Event Reporting System (FAERS) database, along with four observational studies published between 2015 and 2018.
Two studies that were published in JAMA Internal Medicine and BMJ Open in November 2015 found a significantly increased risk of aortic aneurysm or dissection. The studies reportedly found a 124% and a 143% increased risk of aortic aneurysm or dissection. In addition, researchers found that past users had faced a 48% increased risk for these alleged fluoroquinolone side effects.
These studies prompted the FDA to issue this updated warning, which is different from its previous warning in May 2017. In that initial notice, the FDA reportedly stated there was no definitive evidence linking fluoroquinolones to aortic aneurysms despite various patient injury reports.
The updated statement issued by the FDA on Dec. 20, 2018, now notes that there could indeed be a potential correlation with fluoroquinolone side effects and aortic problems.
Overview of Fluoroquinolone Aortic Complications
The data from research sources all indicated an increased risk of aortic aneurysms or dissections, which can be potentially deadly for patients if not promptly treated.
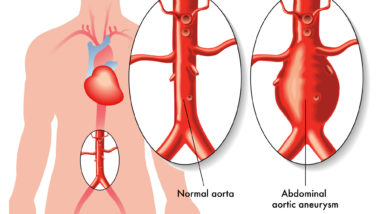
This means that patients prescribed fluoroquinolone antibiotics, like Noroxin or Cipro, should be wary for any signs of aortic aneurysm. Symptoms of this can include throbbing feelings in the stomach, as well as deep pain in the back or the side of the stomach area.
Aortic bulges or aneurysms can form in any section of the aorta, but typically occur in the abdominal area. If not treated, these can evolve into aortic dissection or tears in the inner layer of the aorta. When the inner layer of the aorta tears, it allows blood to separate the inner and middle layers of the aorta, which can also restrict circulation to major organs and can cause heart attack or stroke.
At this point in time, researchers are unsure as to why fluoroquinolone patients may be developing aortic aneurysms or dissection, but they believe it is similar to how the drugs can cause tendon damage. According to research, the treatment mechanism of fluoroquinolones may degrade collagen, causing tendons to weaken. The same effect may damage the aortic walls. This family of drugs has also been linked to liver damage.
Like other fluoroquinolones, Noroxin is prescribed to treat a variety of bacterial infections by inhibiting the bacterial cells’ ability to reproduce. Approximately 20 million prescriptions for fluoroquinolones are filled each year, with many patients unaware of the alleged link with aortic aneurysm or dissection.
The FDA is currently advising fluoroquinolone patients who develop these complications to report them to the FDA MedWatch program to help track the statistics.
Join a Fluoroquinolone Aortic Aneurysm, Aortic Dissection Lawsuit Investigation
If you or a loved one were prescribed Fluoroquinolones such as Cipro, Levaquin or Avelox and were later diagnosed with an aortic dissection or aortic aneurysm, you may have a legal claim. Fill out the form on this page now for a FREE case evaluation or call 1-(855)-JONES-LAW (1-855-566-3752).
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Fluoroquinolone Aortic Aneurysm, Aortic Dissection Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.
Oops! We could not locate your form.