Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.

Fluoroquinolone Basics
Fluoroquinolones are a very common type of antibiotic medication. They include Factive (gemifloxacin), Cipro (ciprofloxacin), Floxin (ofloxacin), Levaquin (levofloxacin), Noroxin (norfloxacin), and Avelox (moxifloxacin). These medications are most often prescribed to treat urinary tract infections and upper respiratory infections.
However, despite the widespread use of these drugs, recent research indicates that patients taking fluoroquinolone antibiotics like Factive may be two to three times more likely to suffer from damage to the aorta, the artery responsible for carrying blood from the heart.
FDA Fluoroquinolone Warning
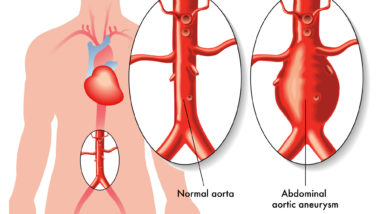
The FDA announced on Dec. 20 that fluoroquinolone drugs, such as Factive, may increase the risk of aortic dissection or ruptured aortic aneurysm, which can lead to dangerous bleeding complications—or even death. These side effects are associated with drugs given either by mouth or through an injection.
Because of these risks, the FDA noted, fluoroquinolone drugs will have new warnings added to their labels and prescribing information. Previously, the FDA had warned that fluoroquinolones like Factive should only be used when deemed completely necessary, with no better options available, because of side effects to the tendons, muscles, joints, and nerves.
“Prescribe fluoroquinolones to these patients only when no other treatment options are available,” the federal agency cautioned. “People at increased risk include those with a history of blockages or aneurysms (abnormal bulges) of the aorta or other blood vessels, high blood pressure, certain genetic disorders that involve blood vessel changes, and the elderly.”
Patients taking fluoroquinolones should be on the lookout for potential ruptured aortic aneurysm or aortic dissection symptoms, which can include sudden, severe, and constant stomach, chest, or back pain, according to the FDA. Unfortunately, symptoms of a rupture aortic aneurysm can appear rather suddenly after the problem has already progressed.
“Be aware that symptoms of an aortic aneurysm often do not show up until the aneurysm becomes large or bursts, so report any unusual side effects from taking fluoroquinolones to your health care professional immediately,” the FDA warned.
Filing a Fluoroquinolone Lawsuit
So far, fluoroquinolone manufacturers have been hit with a slew of lawsuits over serious and even debilitating injuries, alleging that they were not adequately warned about the risks.
If you or a loved one have taken Factive or another fluoroquinolone antibiotic and have suffered from side effects like a ruptured aortic aneurysm, you may be able to file a lawsuit. While filing a lawsuit cannot undo the pain and suffering caused by a ruptured aortic aneurysm, it can at least help to alleviate the financial burden caused by medical expenses and lost wages.
Join a Fluoroquinolone Aortic Aneurysm, Aortic Dissection Lawsuit Investigation
If you or a loved one were prescribed Fluoroquinolones such as Cipro, Levaquin or Avelox and were later diagnosed with an aortic dissection or aortic aneurysm, you may have a legal claim. Fill out the form on this page now for a FREE case evaluation or call 1-(855)-JONES-LAW (1-855-566-3752).
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Fluoroquinolone Aortic Aneurysm, Aortic Dissection Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.
Oops! We could not locate your form.