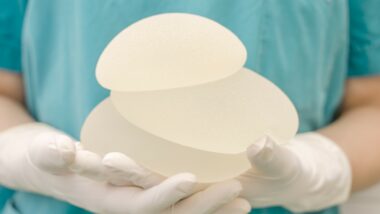
![]() Earlier this summer, several breast implants were recalled by Allergen due to concerns regarding breast implant cancer lymphoma.
Earlier this summer, several breast implants were recalled by Allergen due to concerns regarding breast implant cancer lymphoma.
According to the U.S. Food and Drug Administration (FDA), breast implants may be associated with a rare type of cancer known as breast implant associated anaplastic large cell lymphoma (BIA ALCL). Since the FDA first identified the possible association in 2011, the agency has reportedly received 573 US and global reports of the cancer.
Unlike other breast cancers, breast implant cancer lymphoma begins within the scar tissue capsule that surrounds the implant during the healing process. Luckily, this makes the cancer easy to treat if caught early since the cancerous cells are restricted to the scar capsule. However, if the cancer spreads to the nearby lymph nodes it can be more difficult to treat.
Although foreign regulators have taken a stronger stance against textured implants, the FDA has determined that they don’t have enough information to completely ban the product category. Instead, the agency reportedly called on implant manufacturer Allergan to recall their textured implants related to the cancer.
FDA Announces Allergan Recall
In July 2019, the FDA released a statement informing the public that Allergan would be complying with regulators by recalling several of their textured implants from markets around the world. The recall reportedly includes Natrelle Saline-Filled breast implants, Natrelle Silicone-Filled breast implants, Natrelle Inspira Silicone-Filled breast implants, and Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled breast implants.
Although women with recalled textured implants may be afraid of getting breast implant cancer lymphoma, they are reportedly being encouraged not to remove their implants.
Because of the relatively rate risk of developing BIA ALCL, the FDA has determined that the risk of surgery is not worth it. Additionally, removing the implants before diagnosis does not guarantee that a patient won’t develop the disease.
The FDA has made these recommendations based on research, however some women may still want to get them out. Women whose breast implants were included in the Allergen recall have been directed to speak to their doctor or a board certified plastic surgeon.
Luckily, if women keep their implants in and still develop BIA ALCL, they may be able to recover compensation. According to Spotlight on America, Allergen sent a letter to physicians stating that women who develop BIA ALCL after choosing to keep their implants may be reimbursed for up to $1,000 of diagnostic fees and up to $7,500 surgical fees.
Spotlight reportedly requested an on-camera interview with the company, but their request was denied. Instead, Allergan reportedly gave the following statement:
“Patient safety is a priority for Allergan. We are committed to providing patients, surgeons and healthcare providers with the very latest information about the BIOCELL withdrawal with this new site. It is important to note that the FDA and other health authorities have recommended not to remove or replace textured breast implants or tissue expanders in asymptomatic patients. Patients are advised to speak with a board-certified plastic surgeon about the risks and benefits of their implant type and address any concerns they may have.”
More information about the Allergen recall is available on a dedicated recall website set up by the company.
Join a Free Breast Implants Side Effects Lawsuit Investigation
You may qualify for this breast implant investigation under the following circumstances:
- You were implanted with textured breast implants;
- You were diagnosed with Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA ALCL); and/or
- You’ve suffered from any illness you believe is related to the implants.
Fill out the form on this page for a free case evaluation by a breast implants injury attorney.
This article is not legal advice. It is presented
for informational purposes only.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Breast Implants Side Effects Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
[email protected].