A voluntary Zantac recall has been issued after trace amounts of NDMA (N-Nitrosodimethylamine) was found to have contaminated some batches of the generic and brand name versions of the drug.
A voluntary Zantac recall has been issued after trace amounts of NDMA (N-Nitrosodimethylamine) was found to have contaminated some batches of the generic and brand name versions of the drug.
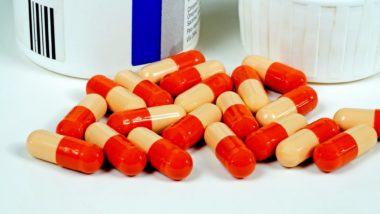
Drug manufacturer Sandoz Inc. has recalled its generic Zantac (ranitidine) capsules in the U.S. after confirming some tested samples were found to be contaminated with NDMA, a potentially carcinogenic substance that is a byproduct of some manufacturing processes.
According to an announcement on the U.S. Food and Drug Administration (FDA) website posted Sept. 23, 2019, Sandoz planned to contact distributors and customers by overnight mail and through its website to arrange for the return of all lots of recalled products, which include 150 mg and 300 mg versions.
Brand name Zantac is made by Sanofi, which has not stopped shipments of the drug to the United States.
In an emailed press release, Sanofi spokeswoman Ashleigh Koss said, “The FDA reported that the levels of N-nitrosodimethylamine (NDMA) in ranitidine in preliminary tests barely exceed the amounts found in common foods.”
She went on to say that the company is conducting its own investigation in addition to working with the FDA.
In the meantime, drug maker Apotex has announced a recall of over-the-counter generic ranitidine sold at Walgreens, Walmart and Rite Aid. The drug maker expressed concerns that the medications could be contaminated with even small amounts of NDMA, which is a contaminant that’s classified as a probable human carcinogen. As of Sept. 28, CVS has also pulled brand name Zantac and the CVS Health brand ranitidine from its shelves.
Zantac (ranitidine) is available in over-the-counter and prescription strengths to help people with ulcers, heartburn and acid reflux issues. The drug is in a class of medications known as histamine-2 blockers, which work by reducing the amount of acid produced in the stomach and also counteracts the corrosive effects of stomach acid that can lead to acid reflux and heartburn.
Animal studies have shown NDMA may be associated with stomach cancer, liver cancer, bladder cancer and colorectal cancer.
Pharmacy Tests Gave Rise to Zantac Recall
Valisure, an online pharmacy and lab based out of New Haven, Conn., initially discovered the NDMA in Zantac and ranitidine. According to USA Today, Valisure found NDMA levels that were quite a bit higher than what is recommended as safe by the FDA.
Valisure’s CEO, David Light, told USA Today that the lab conducted tests to mimic ranitidine’s metabolism process in the stomach. The tests showed that as the drug breaks down, an enzyme produced by the human body might create NDMA as a side effect.
If ranitidine does cause a chemical reaction that produces NDMA inside the body, then the majority of the NDMA the body must eliminate is not just from pills tainted during a manufacturing process, said Light.
Zantac Recall Prompts Lawsuit
At least one class action lawsuit has been filed against Sanofi, which faces allegations that the company and Boehringer Ingelheim Pharmaceuticals knew of the unsafe levels of NDMA in Zantac for years, but concealed the dataf
According to the lawsuit, Valisure used an “FDA-recommended protocol” that found the amount of NDMA in each 150 mg tablet of Zantac to be “more than 26,000 times greater than the amount that can be safely ingested daily.”
The Zantac Recall Lawsuit is Christina Garza et al. v. Sanofi-Aventis U.S. LLC, et al., Case No. 5:19-cv-05772-NC in the U.S. District Court for the Northern District of California.
Join a Free Zantac Cancer Lawsuit Investigation
If you or a loved one was diagnosed with stomach cancer or bladder cancer after taking Zantac or another ranitidine medication, you may qualify to join this Zantac cancer lawsuit investigation. Learn more by filling out out the form on this page for a free case evaluation by a Zantac cancer injury lawyer.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Zantac Cancer Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
[email protected].