Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
An investigation has been launched into Injectafer, an intravenous medication for the treatment of iron deficiency anemia, following patient reports of severe Injectafer side effects. Some patients claim that drugmaker Daiichi Sankyo failed to warn about the risk of hypophosphatemia following an injection with the iron supplement.
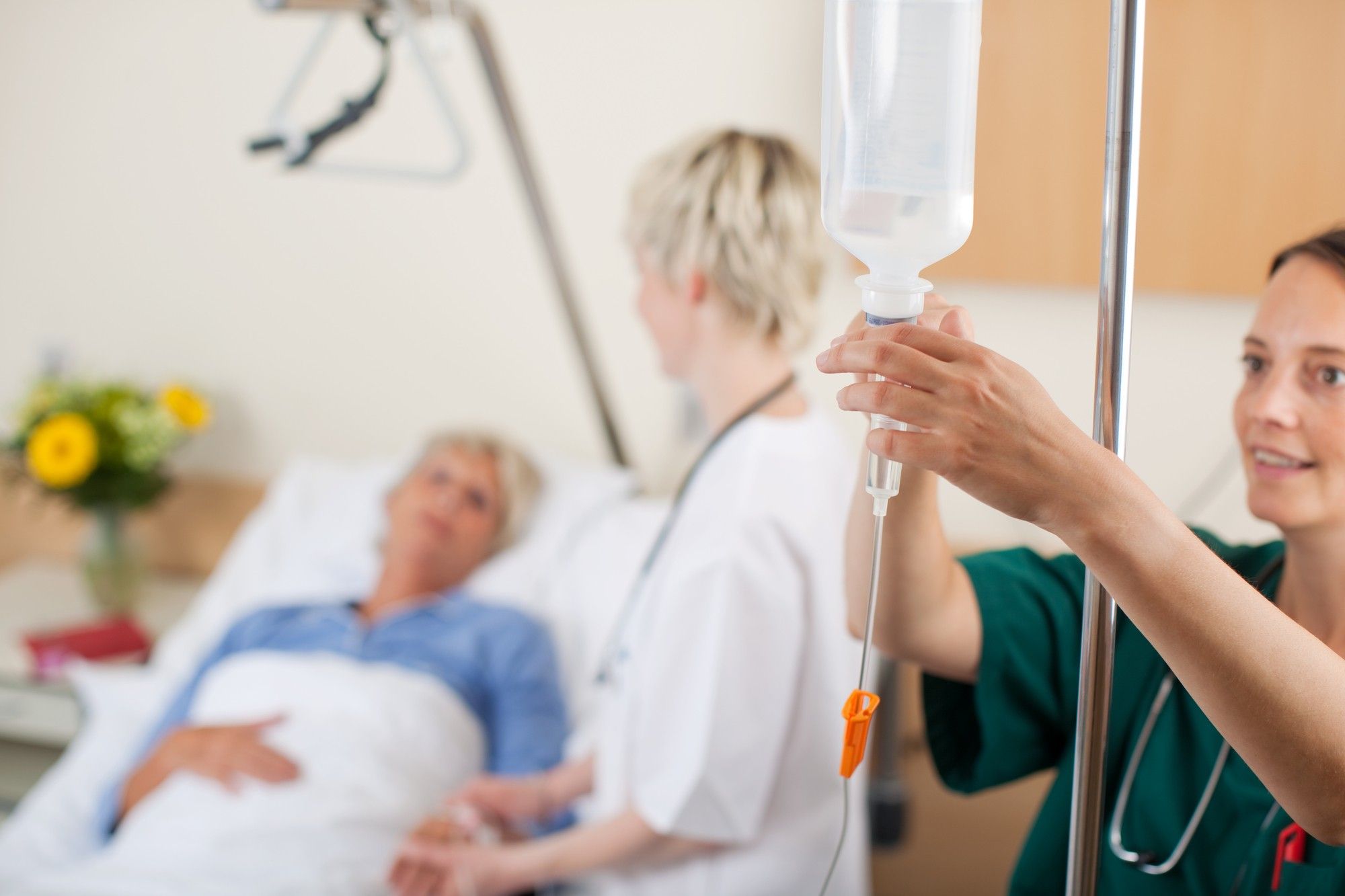
What is an Injectafer Infusion?
Injectafer, also known as ferric carboxymaltose, is a prescription drug prescribed as a treatment for people with anemia or a low red blood cell count due to low iron levels. The drug is given intravenously to patients who have not responded to oral iron supplements.
According to drugmaker Daiichi Sankyo’s website, Injectafer can restore patients’ iron levels to normal after two treatments. It promotes the IV drug for various conditions that cause low iron levels, including chronic kidney disease, certain gastrointestinal issues, heart failure, cancer, and for women who are experiencing low iron levels due to childbirth or heavy periods.
What to Expect After an Injectafer Infusion
The drug’s website notes that Injectafer is often given at an infusion center. It’s given in two doses at least seven days apart. Once the infusion is administered, patients are monitored for 30 minutes to ensure they don’t experience an allergic reaction.
Some patients have reported experiencing severe side effects following an Injectifer infusion.
Injectafer Infusion Side Effects
According to Pittsburgh’s Hillman Medical Center, Injectafer side effects may include flushing, dizziness, nausea, dangerously high blood pressure and low phosphorus levels. In addition, some patients may experience an allergic reaction to the drug, including swelling of the face, tongue, or throat, a rash, fever, chills, difficulty breathing, or a rapid heart rate.
Some patients report that Injectafer’s side effects are much worse than described on the warning label. Several reviewers on EverydayHealth.com claim to have experienced severe hypophosphatemia, or low phosphorus, after being treated with Injectafer.
According to Healthline, hypophosphatemia occurs when there are abnormally low phosphate levels in the bloodstream. Phosphorus is an electrolyte that aids in energy production and nerve function and is vital in building bones and teeth, making it an important component of blood.
Hypophosphatemia can lead to weakness, anorexia, seizures, bone degeneration, coma, and even death. Signs of the condition include fatigue, bone pain or fractures, loss of appetite, irritability, numbness, and confusion, according to Healthline.
“This drug caused me severe hypophosphatemia, landing me in the ER,” wrote one reviewer. “Weeks later I am still trying to stay out of those dangerously low phosphorous levels. Be very careful with this drug as it can be very dangerous! Demand phosphate levels be checked regularly while on it.”
 Injectafer Lawsuits
Injectafer Lawsuits
The Daily Hornet reports that drugmaker Daiichi Sankyo failed to warn patients about the risk of low phosphorus when the drug was approved by the U.S. Food and Drug Administration (FDA) in 2013. In February, the agency reportedly ordered the drugmaker to include the risk of “symptomatic hypophosphatemia” to Injectafer’s warning label following a slew of studies showing a link between the drug and the debilitating condition.
In a clinical study, 75% of the patients who were administered Injectafer developed low phosphorus levels, compared with 8% of those taking a competing iron supplement. Of the Injectafer patients who developed symptomatic hypophosphatemia, 11.3% developed a severe case, reports the Hornet, yet no patients using another drug did so.
Several people have filed Injectafer lawsuits over hypophosphatemia. A Florida woman’s lawsuit claims she developed low phosphorus shortly after being treated with Injectafer for chronic low iron. The suit states that the plaintiff required emergency medical treatment after receiving the injections and she continues to experience unstable phosphorus levels in her bloodstream.
Another patient also reportedly suffered severe Injectafer complications — chest pain, heart palpitations, shortness of breath,body pain, insomnia, and general health deterioration. She says in her Injectafer lawsuit that after receiving the drug in 2016, she developed low phosphorus. The lawsuit against Daiichi Sankyo claims that the complications resulted in the plaintiff being unable to work for more than two months.
The plaintiffs and others claim that the makers of Injectafer ignored studies that linked the medication to low phosphorus levels. Multiple studies conducted between 2013 and 2018 reportedly connected Injectafer to hypophosphatemia. Patients say that the company should have included the risk on its warning label long before February, when the FDA mandated it.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Free Injectafer Harmful Side Effects Lawsuit Evaluation
Fill out the form below for a free case evaluation. If you qualify, a lawyer will contact you to discuss the details of your potential case at no charge to you.

 Injectafer Lawsuits
Injectafer Lawsuits










