 A newly approved PrEP drug may provide relief for patients suffering from Truvada side effects and complications from other HIV drugs.
A newly approved PrEP drug may provide relief for patients suffering from Truvada side effects and complications from other HIV drugs.
On Oct. 3, the U.S. Food and Drug Administration (FDA) approved Descovy (emtricitabine and tenofovir alafenamide) to reduce the risk of HIV in at-risk individuals as a part of the PrEP program. The FDA has only approved one other drug as a part of this program.
According to the Centers for Disease Control (CDC), PrEP stands for pre-exposure prophylaxis, a program that involves daily medicine for people who are at a very high risk for contracting HIV. Studies have shown that PrEP drugs are extremely effective in reducing the risk of getting HIV through sex. When these drugs are taken daily, they reportedly decrease the risk of HIV by 99 percent.
PrEP is recommended for people who are HIV negative and are in an ongoing sexual relationship with an HIV positive partner, according to the CDC. The program may also be recommended for other populations at high risk for HIV.
The addition of Descovy to the PrEP program increases options for people in these at-risk populations.
“PrEP drugs are highly effective when taken as indicated in the drug labeling and can prevent HIV infection,” Jeffrey Murray, deputy director of the Division of Antiviral Products in the FDA’s Center for Drug Evaluation and Research, said in a press release.
“This approval provides more prevention options for certain patients at-risk for acquiring HIV and helps further efforts by the FDA and the U.S. Department of Health and Human Services to facilitate the development of HIV treatment and prevention options to reduce new HIV infections.”
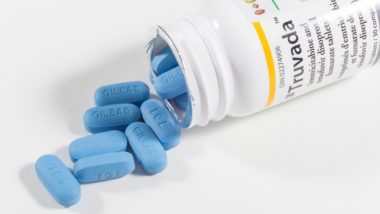
Before the approval of Descovy, only Truvada was approved as part of PrEP. Both drugs are manufactured by Gilead Sciences.
Are Descovy Side Effects Better Than PrEP Truvada Side Effects?
Truvada, the other approved PrEP medication, is associated with serious side effects. Patients have reportedly suffered from bone density and kidney problems.
These PrEP Truvada side effects may stem from the drug’s active ingredient tenofovir disoproxil fumarate (TDF). According to Truvada lawsuits, TDF is not very bioavailable – meaning that high doses of the drug are required for it to be effective. This allegedly results in excess tenofovir ending up in the kidneys and bones of Truvada patients.
Unlike Truvada, the new Descovy medicine uses tenofovir alafenamide – another form of tenofovir which is allegedly more bioavailable. This reportedly makes it less prone to PrEP Truvada side effects.
Tenofovir alafenamide fumarate (TAF) has been the subject of much debate. Lawsuits against Gilead claim that the company knew that TAF was a safer alternative to TDF that posed fewer side effects. Despite this knowledge, Gilead allegedly shelved all research into TAF in order to prolong its profits from patented TDF medications.
Only after the TDF patents were nearing expiration did Gilead allegedly begin researching TAF to replace its expired profits.
Consumers have argued that Gilead prioritized profits over the well-being of patients’ bones and kidneys.
If you or a loved one has suffered from severe bone or kidney side effects while taking an HIV drug containing tenofovir, you may qualify for this HIV medications lawsuit investigation. An HIV drug side effects lawsuit can help to recover damages for medical bills, lost wages, and pain and suffering. Learn more by filling out the free form on this page.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free HIV Medications Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
[email protected].
Oops! We could not locate your form.









One thought on Newly-Approved Drug Should Help PrEP Users Avoid Truvada Side Effects