Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
A group of HIV patients have taken on drug maker Gilead in a lawsuit, saying that the drug maker put profits ahead of patients by selling HIV drugs in a form that was easier to market but more dangerous for the user. The patients also claimed that the company then doubled down on the harm done by these drugs by keeping a safer drug off the market rather than admit that earlier medication could be harmful.
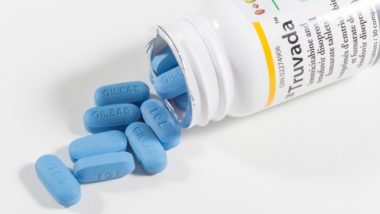
Twenty patients taking HIV drugs including Viread, Truvada, Atripla, Complera, and Stribild have filed a lawsuit against the manufacturer, Gilead. According to the patients, these drugs contain a form of the compound tenofovir that is toxic to patients’ kidneys and bones. Allegedly, the drug company knew that this form of tenofovir could be harmful to the user.
The patients argue that the company opted out of using a less dangerous form of tenofovir that could only be administered intravenously, and instead used the more-dangerous form because it could be administered orally. They say that the company made this choice because oral drugs are easier to sell than intravenous ones.
The Gilead tenofovir drugs lawsuit provides background for the drugs’ development, noting that tenofovir was not first developed by Gilead. Reportedly, it was developed in in the 1980s by European scientists. At the time, it could not be administered orally, and instead, had to be given intravenously. The patients explain that the drug is a nucleotide analogue reverse transcriptase inhibitor (NRTI), which works to block an enzyme that HIV needs to replicate.
Allegedly, Gilead realized that tenofovir given intravenously would not be easy to sell, so the company set about developing a tenofovir that could be taken by mouth. The patients explain that the company was successful in developing an oral formulation, but in so doing, changed the form of the drug. The patients note that the original drug is tenofovir disoproxil, containing a fumaric acid salt, tenofovir disoproxil fumarate (TDF). Allegedly, tenofovir disoproxil fumarate is converted into tenofovir by the patient’s body. The drawback with this drug is that a high dose of the drug — around 300 mg — is needed to make it effective, note the patients.
 Did Gilead Know That the Drugs Posed a Risk?
Did Gilead Know That the Drugs Posed a Risk?
The HIV drug toxicity lawsuit goes on to assert that Gilead knew that the drugs could pose a safety risk to patients. The allegations note that early clinical information indicted that TDF side effects were serious; the drug could allegedly be severely toxic to kidneys and bones. This information was supported by data that the company had previously gained about its other drugs, note the patients. They say that Gilead had produced two other antiviral drugs that had structures similar to tenofovir — cidofovir and adefovir dipivoxil — that had been found to be toxic to kidneys.
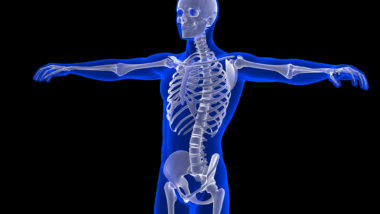
The patients go on to stress that Gilead knew or should have known that TDF side effects could be severe, and could include bone and kidney damage, particularly at the high doses required to be effective. Particularly, they say that “by the time Gilead designed Stribild, it had ten years’ worth of cumulative evidence that TDF injured patients’ kidneys and bones.”
The Gilead HIV drug toxicity lawsuit then states that Gilead not only knowingly sold drugs that were severely harmful to patients, but impeded their replacement with less harmful drugs. Allegedly, the company had developed a safer version of the tenofovir — tenofovir alafenamide fumarate. According to the plaintiffs, this drug is absorbed into the targeted cells more easily than tenofovir disoproxil fumarate, which means that it can be administered at lower doses than its predecessor and be very effective. This, in turn, means that the drug could come with fewer side effects, and less toxicity.
However, the Gilead HIV drugs lawsuit states that Gilead actively abandoned the production of this drug, in an effort not to damage the sales of its tenofovir disoproxil fumarate on the market. The company went so far as to make Viread, Truvada, Atripla, Complera, and Stribild with tenofovir disoproxil fumarate as opposed to tenofovir alafenamide fumarate so as to not have to alert the public to the serious TDF side effects, note the patients.
The company allegedly misrepresented tenofovir alafenamide fumarate as similar to its predecessor in order to not hurt sales of drugs made with tenofovir disoproxil fumarate, when in reality, the two drugs were significantly different, allege the patients.
This is not the first time that Gilead has faced such claims. BioSpace reports on similar allegations. The Los Angeles Times notes that patients in that case argued that patients were needlessly exposed to more than 10 years of TDF side effects while another option should have been available.
The Gilead HIV Drug Toxicity Lawsuit is Case No. Unknown, in the U.S. District Court for the Northern District of California.
Join a Free HIV Medications Lawsuit Investigation
If you or a loved one has suffered from severe bone or kidney side effects while taking an HIV drug containing tenofovir, you may qualify for this HIV medications lawsuit investigation. An HIV drug side effects lawsuit can help to recover damages for medical bills, lost wages, and pain and suffering. Learn more by filling out the free form on this page.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free HIV Medications Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.
Oops! We could not locate your form.


 Did Gilead Know That the Drugs Posed a Risk?
Did Gilead Know That the Drugs Posed a Risk?