 If you have taken Atripla tablet medication for HIV and have suffered complications, you are not alone.
If you have taken Atripla tablet medication for HIV and have suffered complications, you are not alone.
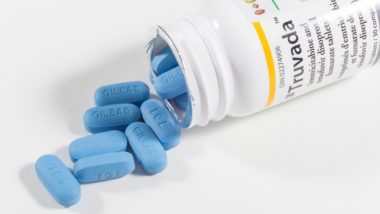
Drug manufacturer Gilead Sciences makes Atripla, Complera, Stribild, Truvada and Viread, all of which contain the active ingredient tenofovir disoproxil fumarate (TDF).
In several lawsuits filed recently, patients allege Gilead knew – but failed to warn – that TDF can cause a decrease in bone density and the onset of kidney problems.
At least one patient accuses Gilead of hiding a less toxic medication for years as the company reaps all possible profits from the more dangerous TDF drugs.
The Atripla tablet and other TDF-based drugs purportedly have low bioavailability, which means the body cannot absorb useful amounts of the active ingredient. As a result, higher doses must be taken by the patient to see a therapeutic effect.
Even though the higher doses of the medication control the HIV virus, patients say their bones and kidneys have been sacrificed in exchange for the drug’s effectiveness.
Atripla Medicine Allegedly Dangerous
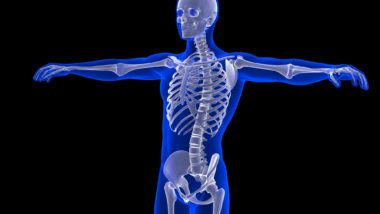
One lawsuit filed by two California men sheds light on the alleged damage that TDF drugs can do. One man who took TDF drugs for 11 years claims he developed osteopenia and osteoporosis in his spine, neck and hip when he was just 35 years old. He was diagnosed with the bone density problems in 2016.
The other gentleman says he developed Fanconi syndrome, a rare kidney disease, after he took the TDF drugs from 2001 through 2011. Fanconi syndrome causes excess amounts of glucose, phosphates, uric acid and potassium to be excreted in the urine. The most common symptoms are weakness and bone pain. He claims he also has been diagnosed with both osteopenia and osteoporosis in the past year.
While alleging that TDF drugs inherently dangerous, the men also accuse Gilead of creating a medication that was less harmful than TDF back in 2000, but choosing not to market the promising drug. Test results on tenofovir alafenamide fumarate (TAF) were allegedly hidden for years.
Gilead purportedly conducted an animal study that showed a lower dose of TAF could be much more effective against HIV because of the drug’s greater potency and potentially better bioavailability. Clinical trials on humans indicated a smaller dose could translate to fewer side effects, but the research results were withheld for years. The lawsuit by the two California men calls the delayed publishing of the results “an act of extreme malice.”
Gilead announced in 2004 that research on TAF had concluded and that the company decided to continue selling the more dangerous TDF drugs, including the Atripla tablet.
TDF-based drugs have been linked to the following adverse side effects:
- Loss of bone mineral density
- Bone death
- Bone fractures
- Osteopenia
- Osteoporosis
- Kidney damage
- Chronic kidney disease
- Kidney failure
- Kidney toxicity
Patients who have suffered any of these or other adverse health conditions due to taking the Atripla tablet or other TDF-based HIV drugs could be eligible to participate in this lawsuit investigation.
If you or a loved one has suffered from severe bone or kidney side effects while taking an HIV drug containing tenofovir, you may qualify for this HIV medications lawsuit investigation. An HIV drug side effects lawsuit can help to recover damages for medical bills, lost wages, and pain and suffering. Learn more by filling out the free form on this page.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free HIV Medications Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
[email protected].
Oops! We could not locate your form.