 Holly and William Angus of Illinois have joined the many other hip replacement sufferers by filing a Stryker lawsuit on September 4, 2013. They’re joining a quickly growing multidistrict litigation (MDL) movement against Stryker, which means they’ll enjoy a faster resolution to their complaint while still receiving an individual trial by jury.
Holly and William Angus of Illinois have joined the many other hip replacement sufferers by filing a Stryker lawsuit on September 4, 2013. They’re joining a quickly growing multidistrict litigation (MDL) movement against Stryker, which means they’ll enjoy a faster resolution to their complaint while still receiving an individual trial by jury.
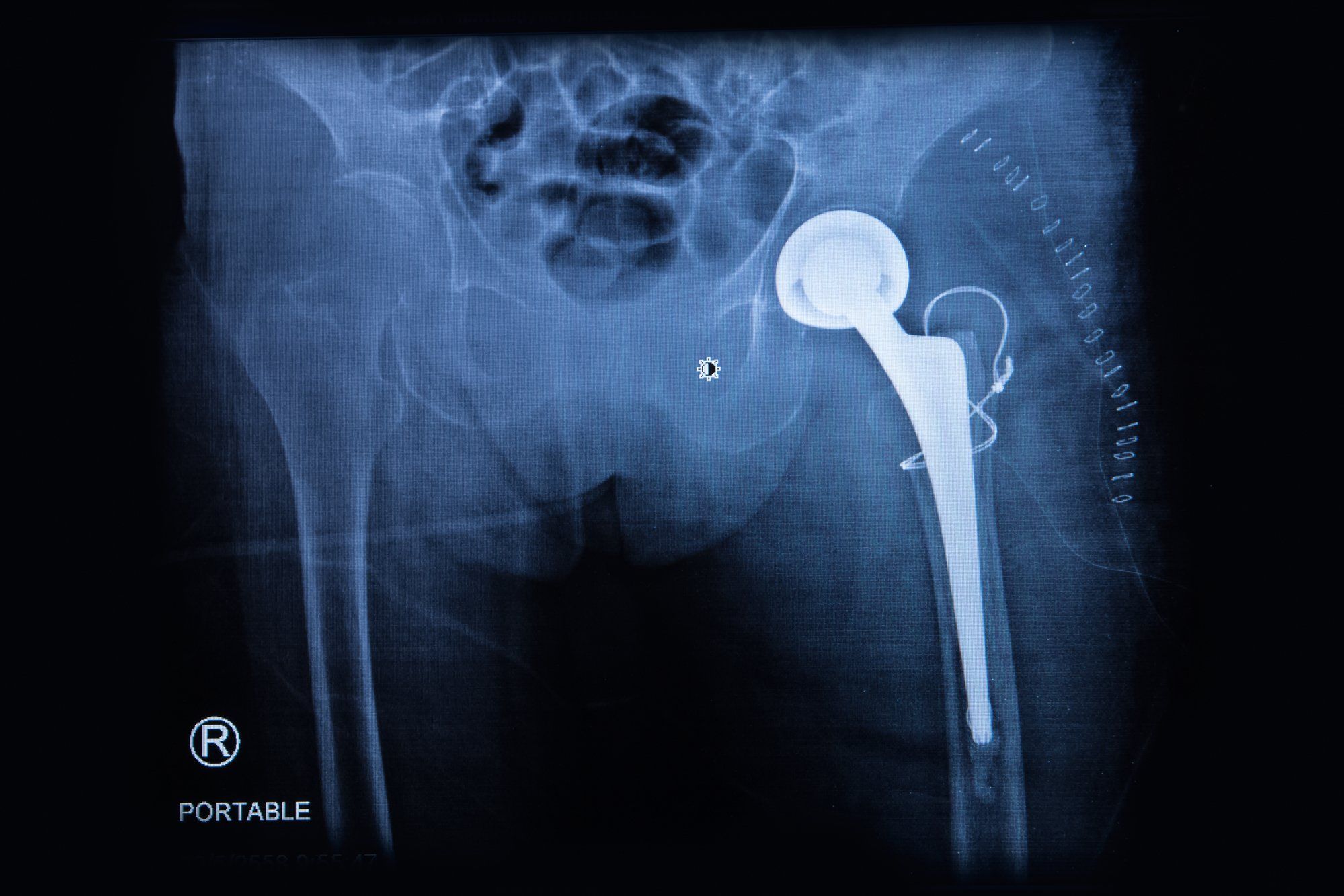
Howmedica Osteonics is the maker of the allegedly defective Stryker hip replacement system, which isn’t “technically” a metal on metal hip implant, but it does have metal on metal components. Holly was the recipient of a Stryker implant, and thought she was getting a safe and effective device.
“In February of 2009, Defendants released the Rejuvenate Modular Primary Hip System (‘Rejuvenate’ or the ‘Product’), which was approved through the FDA’s 510(k) process on or about June 3, 2008. The Rejuvenate system is a dual modular hip replacement prosthesis. It is intended for patients requiring primary total hip arthroplasty or replacement due to joint disease of the hip resulting from non-inflammatory degenerative arthritis,” the lawsuit states.
According to the Illinois couple’s Stryker lawsuit, Howmedica “claims that laboratory testing demonstrates the compatibility of these materials without fretting and corrosion. Defendants also claim that this metal alloy has been tested and proven by Defendants to resist the effects of corrosion and fretting. Despite Defendants’ claims, this combination of materials has been reported to cause fretting and corrosion. Since the 1980s, medical and scientific literature has reported corrosion to be a problem when (titanium) and (cobalt chromium) have been used at modular junctions in medical implants. Upon information and belief, Defendants represented and warranted in its marketing and sale of the Rejuvenate that its proprietary materials alleviate these problems.”
However, Stryker hip implants also allegedly fret, erode and have been linked to metal poisoning, which can be life-threatening.
“In April of 2012, Defendants issued an Urgent Field Safety Notice to surgeons and hospitals in the United States regarding the Rejuvenate. In this Urgent Field Safety Notice, Defendants acknowledged that it had received reports of device failure due to heavy metal contamination. The Urgent Field Safety Notice specifically referred to failures at the taper neck junction between the neck and stem due to corrosion and fretting,” the Stryker hip lawsuit says.
However, this safety notice came too late for Holly, who claims she was already experiencing the dangerous Stryker side effects. “In the Urgent Field Safety Notice, Defendants went on to describe symptoms and findings identical to those experienced by Plaintiff. Among those symptoms and findings specifically mentioned in the Urgent Field Safety Notice were tissue necrosis, metallosis, adverse soft tissue reaction, and pseudotumor formation.”
“On October 24, 2011, Plaintiff Holly Angus underwent left total hip replacement surgery performed by Dr. Thomas Mulvey, at OSF St. Francis Medical Center in Peoria, Illinois. Plaintiff was implanted with a Rejuvenate Modular stem, neck and other Stryker products. Subsequent to implantation of the Rejuvenate hip, Plaintiff Holly Angus began experiencing discomfort, and a diagnostic workup revealed elevated levels of the metal ions cobalt and chromium.”
Her Stryker hip implant was allegedly poisoning her, but at least the damage was caught in time. Due to a faulty hip implant, she says she “has suffered, and continues to suffer, both injuries and damages including, but not limited to, past, present and future physical and mental pain and suffering; and past, present and future medical, hospital, rehabilitative and pharmaceutical expenses, and other related damages.”
Standing Together
Holly’s husband qualifies to join the Stryker MDL to seek damages for loss of consortium. The couple claims that Howmedica “failed to timely report adverse events regarding the Rejuvenate; failed to timely conduct failure investigations and analysis; failed to timely report any and all information concerning product failures and corrections; failed to timely and fully inform FDA of unanticipated adverse effects, increases in the incidence of adverse effects, or device failures necessitating a labeling, manufacturing or device modification; failed to conduct necessary design validation; and, sold a misbranded and adulterated product.”
The Illinois couple is suing the company for negligence, negligence per se, failure to warn, design defect, manufacturing defect, negligent misrepresentation, breach of warranty and loss of consortium. Holly is seeking all damages, cost of expenses, interest, and any other relief the court sees as appropriate.
The Stryker Hip Implant MDL is In Re: Stryker Rejuvenate and ABG II Hip Implant Products Liability Litigation, MDL No. 2441, in the U.S. District Court for the District of Minnesota.
Did You Undergo Hip Replacement Surgery?
If you are one of the many patients who received a Stryker hip implant, you probably qualify for a Stryker legal claim. You can find out more about your options at the DePuy, Biomet, Wright Medical Technology & Others, Metal on Metal Hip Implant Class Action Lawsuit Investigation. Once you submit your information, an attorney will contact you if you qualify for a free Stryker claim review.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.











One thought on Couple Files Stryker Hip Replacement Lawsuit
How can i find out what hip replacement material was used on me?