 Arkansas plaintiff Sarah Nail is suing Stryker Corporation for the injuries she allegedly sustained from their metal-on-metal hip replacement Rejuvenate system. Nail alleges that she suffered from device failure, tissue infection, and malposition of the device.
Arkansas plaintiff Sarah Nail is suing Stryker Corporation for the injuries she allegedly sustained from their metal-on-metal hip replacement Rejuvenate system. Nail alleges that she suffered from device failure, tissue infection, and malposition of the device.
Initially, Nail underwent surgery to completely replace her left hip on May 31, 2011, and had chosen the Rejuvenate System as her hip module. Soon after the device was implanted, Nail started experiencing extreme pain and discomfort, including an area of the hip module that refused to rotate, causing her throbbing pain throughout her pelvis. Medical diagnostics revealed that at some point after implantation, the device had loosened inside the patient causing malposition and infection.
To stop the pain and remedy her condition, Nail underwent revision surgery on September 12, 2012. The Rejuvenate system was removed from her body, and was required to go in for another surgery sometime later to correct the device’s left over problem. Nail still requires frequent follow-up care, and has been incurring medical bills since her surgeries.
At no point in time was Nail or her surgeons were advised that these side effects were not possible, nor had any special instructions been left for the surgeon to follow. Had Nail known about these possibilities, she never would have gotten the Rejuvenate system.
So for manufacturing, selling, distributing, and marketing a dangerous device, Nail is suing Stryker Corporation. The charges include: negligence, false advertising, concealing information, and misrepresenting a product.
Overview of Stryker Rejuvenate Complications
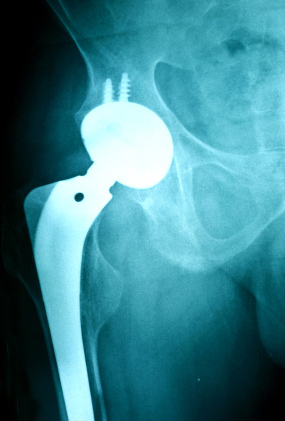
Stryker Corporation released the Rejuvenate system after the FDA approved it in February 2009. The Rejuvenate system consists of two components: the chromium-cobalt neck and a titanium stem. These parts act as a substantial ball-and-socket joint that is meant to be inserted into the damaged pelvic region, which is vital for lower body mobility. What makes the Rejuvenate system different from other metal-on-metal hip modules is that the titanium alloy that it is made of was specifically engineered to be resistant to fretting and corrosion. These issues have been the biggest concerns amongst medical experts, due to the risk of either side effect learning to blood metal poisoning.
Additionally, Stryker boasted that this alloy was stronger and less rigid than other titanium alloys, and was even flexible enough for the metal hip module to be specifically fitted for each individual patient. Despite being engineered against corrosion and fretting, Stryker still found itself being at the center of many lawsuits that their competitors were facing. In April 2012, Stryker sent out an Urgent Field Safety Notice to surgeons and hospitals around the country regarding the Rejuvenate. The warning stated that there had been numerous injury reports indicated device failure and heavy metal poisoning in the blood.
The company admitted in the notice that the metal poisoning was due to the ball-and-socket joint rubbing against one another during the body’s movements; causing the corrosion and fretting. Eventually, Stryker cooperated with the FDA when the agency initiated a Class II recall of the Rejuvenate system.
Stryker Rejuvenate Litigation Movement
While the recall was helpful, it came too late to many patients; Nail was one of these patients. So along with several other patients who have suffered similar injuries as her, Nail is joining a multidistrict litigation (MDL) suit. This MDL is currently still pending.
The Stryker hip MDL is In re: Stryker Rejuvenate and ABG II Hip Implant Products Liability Litigation, MDL No. 2441.
This individual case is labeled as: Sarah Nail v. Stryker Corporation, Case No. 0:13-cv-02285-DWF-FLN, in the United States District Court of Minnesota.
File a Stryker Rejuvenate Lawsuit Today
If you believe that you or a loved one have been the victim of a metal hip injury, you have legal options. Please visit the Stryker Metal Hip Implant Recall Class Action Lawsuit Investigation. There, you can submit your claim for a free legal review and if it qualifies for legal action, a seasoned metal hip lawyer will contact you for a free, no-obligation consultation. You will be guided through the litigation process at no out-of-pocket expenses or hidden fees. The metal hip attorneys working this investigation do not get paid until you do.