The FDA warns patients that a common antibiotic family, fluoroquinolones, may cause aortic aneurysm symptoms.
The FDA warns patients that a common antibiotic family, fluoroquinolones, may cause aortic aneurysm symptoms.
Fluoroquinolones are a family of common antibiotics, used to treat bacterial infections from respiratory infections to urinary tract infections. Many people have taken a fluoroquinolone antibiotic at some point in their lives, and may not have realized it. In fact, over 20 million people are prescribed fluoroquinolone antibiotics each year. And many of these people may not know about the dangers of aortic aneurysm symptoms.
If you have been given antibiotics for a range of infections, including bacterial bronchitis, bacterial gastroenteritis, pneumonia, pelvic inflammatory disease, sinusitis, septicemia, intra-abdominal infections, joint and bone infections, soft tissue and skin infections, typhoid fever, or others, you may have been given a fluoroquinolone.
Fluoroquinolone drugs include:
- Avelox
- Cipro
- Factive
- Floxin
- Levaquin
- Noroxin
Though the drugs are widely useful, they may come with serious side effects that could harm patients.
Research suggests that fluoroquinolone drugs like Noroxin and others may cause problems like aortic aneurysm symptoms. Reportedly, people who take these drugs are two to three times more likely than the average person to experience problems relating to their aorta.
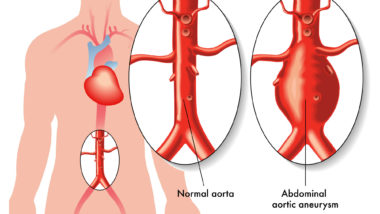
The aorta is the heart’s main artery that carries oxygenated blood to the body. Damage to the aorta can be serious and even fatal.
Two studies published in 2015 suggest that fluoroquinolone use may be connected to aortic aneurysm symptoms and other damage to the aorta, which can cause serious internal bleeding into the abdominal cavity.
According to the research, the drugs can cause the collagen in the aorta to break down. This can cause the walls of the aorta to weaken and rupture. This can cause blood to flow between two layers of the walls, causing an aortic aneurysm, or a bulge in the wall. In other cases, the aortic wall can break entirely, in a case of aortic dissection.
This isn’t the first time that researchers have discovered that fluoroquinolone antibiotics can weaken collagen. Earlier research showed that the drugs could weaken tendons because it degraded the collagen that gives tendons their strength.
According to the 2015 studies into fluoroquinolone drugs’ effect on heart health, people who currently take fluoroquinolone have between a 124 and 143 percent increased risk of aortic dissection or an aneurysm than those not taking fluoroquinolone.
People who do not currently take fluoroquinolone but had once taken it at least 60 days before having aortic aneurysm symptoms were found to also have a significantly higher risk of suffering aortic aneurysm symptoms. Though their risk was lower than current fluoroquinolone users, past users had a 48 percent increased risk of developing aortic aneurysm symptoms.
Up until May 2017, the FDA said that they did not have enough research into the risks associated with fluoroquinolone use to make a warning about aortic risks. However, the FDA recently changed its stance. On Dec. 20, 2018, the FDA issued a statement about both aortic dissection and aortic aneurysm symptoms in association with fluoroquinolones like Noroxin and Cipro.
According to the FDA, this updates stance came after the agency received numerous patient claims of an aortic aneurysm and dissection after use of drugs like Noroxin, and after new research on the drugs was produced.
Join a Fluoroquinolone Aortic Aneurysm, Aortic Dissection Lawsuit Investigation
If you or a loved one were prescribed Fluoroquinolones such as Cipro, Levaquin or Avelox and were later diagnosed with an aortic dissection or aortic aneurysm, you may have a legal claim. Fill out the form on this page now for a FREE case evaluation or call 1-(855)-JONES-LAW (1-855-566-3752).
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Fluoroquinolone Aortic Aneurysm, Aortic Dissection Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
[email protected].
Oops! We could not locate your form.