Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
Levaquin is one of a class of antibiotics known as fluoroquinolones, which have been implicated in an elevated risk of serious damage to the aorta.
With over 20 million prescriptions written for these common antibiotics every year, it is possible that a significant number of people are at risk for Levaquin aortic aneurysm injuries.
The FDA’s warning regarding fluoroquinolones and the danger of aortic aneurysm injuries was originally published in May 2017, and updated on Dec. 20, 2018. Since the warning was published, the FDA has required a black box warning regarding the risk of aortic tears.
A Brief History of Fluoroquinolone Antibiotics
Fluoroquinolones are broad spectrum antibiotics used for both human patients and animals in veterinary medicines.
The predecessor of these medications, nalidixic acid, was first introduced in the early 1960s and used to treat infections of the urinary tract. Since that time, over 10,000 similar compounds have been developed, but very few are used today.
Fluoroquinolones represent the fourth generation of the quinolone family of medications. For many years, it was the first line treatment for many common infections, including those of the sinuses and respiratory system.
However, problems became apparent in the early part of the century. In July, 2008, the FDA added a Black Box warning about the elevated risks of tendon inflammation and rupture.
In early 2011, another warning was issued to patients suffering from a muscular disorder known as myasthenia gravis. Only two years later, a third warning was added, advising patients of potential nerve damage risk in the extremities (peripheral neuropathy).
In July, 2018, the FDA announced that fluoroquinolone drugs had been associated with severe hypoglycemia (dangerous drops in blood sugar levels) and may also have an impact on mental health.
Fluoroquinolones and Levaquin Aortic Aneurysm
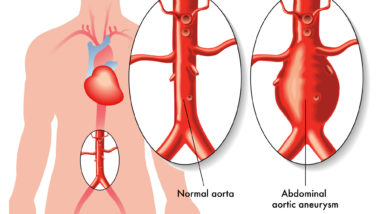
Current concerns about Levaquin aortic aneurysm were raised after the FDA received adverse event reports of patients who had experienced a rupture, or tear in the primary artery that supplies blood from the heart to the rest of the body.
Although it is not yet understood why this happens, the FDA has estimated that as many as three out of 1,000 patients could be at risk for aortic aneurysm.
The FDA advises that patients with a previous history of aneurysm as well as those who may be suffering from atherosclerosis (hardening of the arteries), hypertension, Marfan Syndrome or Ehlers-Danlos syndrome avoid taking fluoroquinolones.
Other Fluoroquinolone Drugs
In addition to Levaquin, members of the fluoroquinolone class of antibiotics include:
- Avelox
- Cipro
- Factive
- Floxin
- Noroxin
Any or all of these could result in injuries similar to Levaquin aortic aneurysm. While these drugs do pose risks, patients who have been prescribed a fluoroquinolone should consult with their doctor before they stop taking the medication.
Join a Fluoroquinolone Aortic Aneurysm, Aortic Dissection Lawsuit Investigation
If you or a loved one were prescribed Fluoroquinolones such as Cipro, Levaquin or Avelox and were later diagnosed with an aortic dissection or aortic aneurysm, you may have a legal claim. Fill out the form on this page now for a FREE case evaluation or call 1-(855)-JONES-LAW (1-855-566-3752).
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Fluoroquinolone Aortic Aneurysm, Aortic Dissection Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.
Oops! We could not locate your form.