Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.

According to Forbes, the FDA issued its most recent warning regarding fluoroquinolone antibiotics side effects on Dec. 20, 2018, stating the antibiotics could increase the chances of aortic aneurysm and dissection.
The agency issued this warning after receiving numerous injury reports from patients and four observational studies. The FDA went on further to state that more than one study indicated that fluoroquinolone antibiotics more than doubled the risk of aortic injuries compared to other antibiotics.
“The results of all four studies provide consistent evidence of an association between fluoroquinolone use and aortic aneurysm or dissection,” the FDA said.
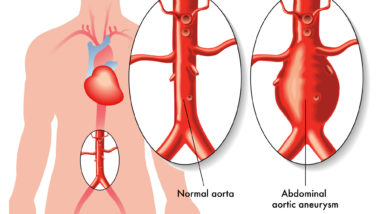
An aortic aneurysm is an unusual bulging or enlargement of the aorta, the body’s main artery, which stems from the top of the heart and extends down to the abdomen in front of the spinal cord.
Aortic tears or ruptures are life threatening complications that interfere with the body’s ability to carry blood out of the heart. Aortic dissections occur in the inner layer of the aorta, which allows for blood to enter the aortic wall and causes the middle and inner layers of the aorta to separate.
This can significantly decrease blood supply to major organs, which can cause life threatening heart attacks or stroke along with other serious problems.
It is important to note that this most recent warning is different than an earlier warning released in May 2017, which stated the FDA did not have definitive proof that an increased risk of aortic aneurysm was linked to fluoroquinolone antibiotics side effects even with several patient injury reports.
The FDA and the rest of the medical community have been concerned for years over this alleged correlation, with even earlier studies finding that fluoroquinolone antibiotics can cause tendon rupture. It is believed fluoroquinolones could damage the aortic wall in a similar fashion, with the FDA issuing warnings for this complication in July 2008.
Overview of Fluoroquinolone Antibiotics Side Effects
Two studies published in 2015 indicated that fluoroquinolone antibiotics could damage collagen, which could lead to aortic tissue damage and eventually evolve into aortic aneurysm or dissection.
These studies were published in JAMA Internal Medicine and BMJ Open in November 2015 and found that fluoroquinolone patients faced a 124 percent and 143 percent increased risk of developing an aortic aneurysm or dissection.
Fluoroquinolones have been on the market for over 30 years, and are used to treat a wide range of bacterial infections. It is estimated that over 20 million fluoroquinolone prescriptions are written each year to treat common bacterial infections, but their recent association with aortic aneurysms and dissection has left many wary of the medications.
Antibiotics in the fluoroquinolone drug class include, but are not limited to:
- Avelox (moxifloxacin)
- Cipro (ciprofloxacin)
- Factive (gemifloxacin)
- Floxin (ofloxacin)
- Levaquin (levofloxacin)
- Noroxin (norfloxacin)
Join a Fluoroquinolone Aortic Aneurysm, Aortic Dissection Lawsuit Investigation
If you or a loved one were prescribed Fluoroquinolones such as Cipro, Levaquin or Avelox and were later diagnosed with an aortic dissection or aortic aneurysm, you may have a legal claim. Fill out the form on this page now for a FREE case evaluation or call 1-(855)-JONES-LAW (1-855-566-3752).
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Fluoroquinolone Aortic Aneurysm, Aortic Dissection Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.
Oops! We could not locate your form.