Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.

In December 2018, the U.S. Food and Drug Administration (FDA) announced that research showed that Cipro, Avelox, and other drugs in the fluoroquinolone antibiotic class may be associated with life-threatening aortic complications.
“A U.S. Food and Drug Administration (FDA) review found that fluoroquinolone antibiotics can increase the occurrence of rare but serious events of ruptures or tears in the main artery of the body, called the aorta. These tears, called aortic dissections, or ruptures of an aortic aneurysm can lead to dangerous bleeding or even death,” the FDA said in a safety alert.
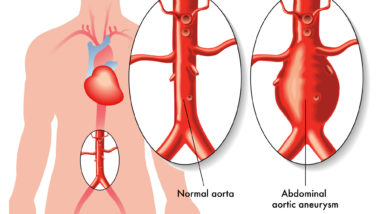
The aorta is the largest artery in the body and carries oxygenated blood from the heart to the rest of the body. When the multi-layer wall of the aorta is weakened, it can create a bulge which is called an aortic aneurysm.
Although aortic aneurysms are not inherently life-threatening, they can quickly lead to other, more dangerous conditions. For example, the flow of blood can increase the weakness in the aortic wall, leading to a complete tear of the aorta known as aortic dissection. This can lead to blood pooling in the chest and abdomen, insufficient blood flow to vital organs, and other life-threatening complications if not treated immediately.
“Be aware that symptoms of an aortic aneurysm often do not show up until an aneurysm becomes large or bursts, so report any unusual side effects from taking fluoroquinolones to your health care professional immediately,” the FDA warned.
Fluoroquinolones such as Avelox and Cipro are broad-spectrum antibiotics commonly prescribed to treat bacterial infections including bacterial bronchitis, pneumonia, sinusitis, septicemia and intra-abdominal infections, joint and bone infections, soft tissue and skin infections, typhoid fever, bacterial gastroenteritis, urethral and gynecological infections, pelvic inflammatory disease, and more.
Following the results of the FDA review which revealed the increased risk, the agency will reportedly be requiring new risk guidance on fluoroquinolone labels. These warnings will address these side effects and other fluoroquinolone side effects involving tendons, muscles, joints, and the nervous system.
Additionally, the risk of Cipro side effects will change how doctors prescribe these medications. The FDA states that individuals at a high risk for aortic events should not be prescribed fluoroquinolones, if avoidable.
“Fluoroquinolones should not be used in patients at increased risk unless there are no other treatment options available,” the FDA warned. “People at increased risk include those with a history of blockages or aneurysms (abnormal bulges) of the aorta or other blood vessels, high blood pressure, certain genetic disorders that involve blood vessel changes, and the elderly.”
The FDA’s recent announcement is a change from the agency’s previous statement in May 2017. At that time, the agency stated that it lacked sufficient evidence to confirm a link between fluoroquinolones and aortic injuries. The change in stance was reportedly due to a review of patient reports and four observational studies.
Join a Fluoroquinolone Aortic Aneurysm, Aortic Dissection Lawsuit Investigation
If you or a loved one were prescribed Fluoroquinolones such as Cipro, Levaquin or Avelox and were later diagnosed with an aortic dissection or aortic aneurysm, you may have a legal claim. Fill out the form on this page now for a FREE case evaluation or call 1-(855)-JONES-LAW (1-855-566-3752).
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Fluoroquinolone Aortic Aneurysm, Aortic Dissection Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.
Oops! We could not locate your form.