Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
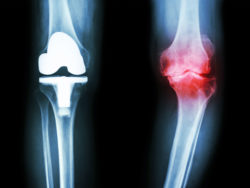
Knee replacement surgery is becoming more common in the United States, with around 4.4 million Americans having undergone knee replacement surgery. A wide variety of knee replacement implants are available for the millions of patients needing knee arthroplasty surgery.
However, not all knee replacement implants are made of the same quality. There have been several recalls by implant manufacturers due to design or construction problems. Some recalls affect only one component of an implant, while others affect the entire device.
One of these recalls was initiated in December 2015 and affected the Arthrex iBalance’s TKA tibial tray. Arthrex described this implant as a “a complete, minimally invasive instrument and implant platform for the treatment of multicompartmental knee cartilage degeneration as a result of osteoarthritis or post-traumatic arthrosis in the medial, lateral and patellofemoral compartments of the knee.”
Tibial trays in knee replacement implants are attached to the tibia and comprise the lower part of the implant. According to an FDA statement, in the Arthrex iBalance, the tibial tray had a smooth outer surface that made the component incompatible with previous models that used a rough texture. Due to these inconsistencies, over 2,000 units were recalled by Arthrex.
According to consumer complaints, some Arthrex knee arthroplasty patients have experienced complications due to the defects that prompted the recall. Some Arthrex knee arthroplasty patients have reportedly even required revision surgery to fix the issue.
Another knee implant which has been subject to consumer complaints is the Exactech Optetrak knee implant. A study published in Orthopaedics & Traumatology: Surgery & Research revealed that a shocking number of patients were dissatisfied with their implant or were experiencing complications allegedly related to their Exactech knee replacement system.
The analysis of 110 protheses in 106 patients 25 months after initial surgery revealed that 15 percent of patients were disappointed with the implant, 22 percent experienced pain, 22 percent of patients suffered from the beginnings of tibial loosening, and 13 implants needed revision surgery due to the issues.
Symptoms of knee implant failure may include pain, mobility issues, swelling, instability problems, infections, fractures, disassociation of the implant, inflammation, loosening of implant, change in component position, kneecap moving out of place, and other symptoms.
As some consumers have reported, revision surgery may be necessary to resolve problems with an Arthrex iBalance knee implant or another implant that caused complications. During revision surgery, a surgeon will remove the implant causing problems and replace it with a new device.
Although the process sounds simple, the surgery can be complicated by a variety of factors. If the failed implant has caused damage to the surrounding tissue and bone, a surgeon needs to be very careful when replacing the device so that they do not cause further damage. If the damage is so severe than bone needs to be removed, the surgery may also involve the process of grafting new bone so that the implant can be fixed into place.
Join a Free Knee Replacement Class Action Lawsuit Investigation
If you or a loved one suffered from complications caused by an Arthrex knee implant or an Exactech knee implant, you may have a legal claim. Get help now by filling out the form on this page for a FREE case evaluation.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Knee Replacement Class Action Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.
Oops! We could not locate your form.












