Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
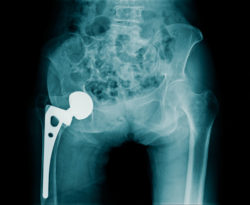
The DePuy recall withdrew 93,000 ASR metal on metal hip implants from around the world, including 37,000 implants in the United States alone.
The DePuy ASR hip replacement recall was reportedly initiated after the company was made aware of data from a United Kingdom joint registry. The registry found that 13 percent of patients who opted for the DePuy ASR hip implant required revision surgery due to serious metal hip implant complications.
In addition to the registry, DePuy received numerous reports indicating increased risks of cancer and degenerative heart disease allegedly as a result of the defective nature of the metal-on-metal hip implant.
Since 2008, the FDA has received up to 400 injury reports indicating problems with the DePuy ASR metal hip implant. By that point, it was only three years after the device’s approval in 2005.
Overview of DePuy ASR Metal Hip Implant Complications
The DePuy ASR hip implant was approved through the FDA’s 510(k) program, which allows a medical device to enter the market without extensive preclinical testing. The medical device can qualify for this program as long as it works on an equivalent level with a similar product already on the market.
Even though this program quickly allows much needed medical devices to enter the market, critics say it often causes products without a complete safety profile to be released. Many patients required revision surgery to fix DePuy ASR hip implant complications, which often require longer recovery times and more expensive medical bills.
Years after the ASR hip replacement recall, Johnson & Johnson and DePuy are still facing fallout from customers. Many patients who required revision surgery have opted to file legal action against the company for allegedly failing to warn them against the defective nature of their device.
Like other metal-on-metal hip implants, DePuy ASR hip implants were marketed to be superior choices for patients needing a hip prosthetic. When metal-on-metal hip implants were first released into the market, they were thought to be overtly superior to plastic or ceramic models.
So what makes metal-on-metal hip implants so problematic? Initially, they were invented as an efficient alternative to their ceramic and plastic counterparts. Believed to last longer and possess better durability, they were thought to be all-around superior at the time. However, not long after metal hip implants were released, problems with the metal-on-metal design were reported.
It was soon discovered that, as the metal ball-and-socket joints rubbed together as the patient moved, metal particles started rubbing off from the device and slipped into the patient’s bloodstream, leading to metal poisoning, pain, implant loosening, device failure, and revision surgery. This often can compound the patient’s condition and cause them to suffer a multitude of other problems including:
- Debilitating pain
- Corrosion
- Fretting
- Infection
- Limited movement
- Formation of pseudotumors and non-cancerous pseudotumors
- Tissue death
- Loss of bone structure or strength (can result in bone fractures)
- General hypersensitivity reaction (skin rash)
- Cardiomyopathy
- Neurological changes including sensory changes (auditory or visual impairments)
- Psychological status change (including depression or cognitive impairment)
- Renal function impairment
- Thyroid dysfunction (including neck discomfort, fatigue, weight gain or feeling cold)
These problems have reportedly forced patients to undergo revision surgery to resolve them, which eventually led to the DePuy ASR hip recall in 2010 and ultimately a massive settlement fund to resolve thousands of product liability claims.
DePuy and Johnson & Johnson are currently facing thousands of DePuy ASR hip implant lawsuits from patients who allegedly suffered device complications.
DePuy ASR Recall
DePuy voluntarily recalled the ASR XL hip implants in August 2010. A study conducted in England found the implants failed at alarmingly high rates. The five-year failure rate of the implant was reportedly found to be 13 percent. This means that the ASR XL hip implant was found to fail within five years in 1 out of every 8 patients.
Although DePuy initially protested a formal recall, the device was recalled shortly after the study’s results were released.
“As part of our ongoing post-market surveillance of all products, DePuy is continually evaluating data from a variety of sources including national joint replacement registries, published literature, company sponsored clinical trials, internal complaints data and unpublished clinical research reports,” the company states in the official FDA recall.
The recall notice recommended that patients follow up with a doctor at least annually for the first five years after they undergo revision surgery. For patients with symptoms of implant failure, the FDA recall recommended that doctors investigate the levels of cobalt and chromium ion levels in a patient’s blood and/or perform MRI and ultrasound scans to survey the joint. If metal ions were found to be above 7 parts per billion, the FDA recommends a second test three months later in order to “identify patients who require closer surveillance” and further imaging. If imaging reveals complications such as “soft tissue reactions, fluid collections or tissue masses”, the FDA notes that revision surgery “should be considered”.
Other DePuy Recalls
Due to concerns that metal hip replacements were unsafe, the FDA proposed a regulation change for metal-on-metal hip implant manufacturers: the companies would have to prove the products were safe before distributing them. Unfortunately for the companies, it would potentially force them to seek higher approval and disclose the risk of deficiencies with the product.
After the ASR recall, DePuy previously that it would stop selling its metal-on-metal hip implants and would only be selling hip replacement systems made from ceramic or plastic. In DePuy’s announcement regarding the discontinuation of their metal hip implant products, DePuy stated that the devices accounted for less than 2 percent of implants sold in the United States and Europe last year, marking a 90 percent drop in their popularity since 2007.
DePuy Orthopaedics said it will discontinue its Ultamet metal-on-metal hip implants and complete ceramic-on-metal hip implants. DePuy had been distributing these products internationally since August 31, 2012. The company had chosen to export due to the low use of the products in the U.S., competing goods, and the proposed regulation changes by the FDA.
Although the withdrawal came at a time of legal action and regulatory scrutiny, DePuy stressed the fact that the decision was not actually a true “recall” because it was not prompted by safety concerns.
“The decision to discontinue these products is not related to safety or efficacy, and is not a recall,” DePuy said in a statement. “Ultamet and Complete are backed by clinical data showing they are safe and effective options for patients who are candidates for hip replacement.”
Despite company assurances about the safety of their metal on metal hips, some consumers remained skeptical of the company’s metal on metal hip products.
Metal-on-metal hip implants had been previously under to the agency’s 510(k) clearance process, which only requires that the company manufacture a device that is at least almost equivalent to an already approved one. Following consumer safety concerns, Consumers Union sent a letter to the FDA expressing their approval for the proposal. The group had added that the FDA should push manufacturers to pull the metal-on-metal hip implants from the market given the high risk or injuries they pose to patients, as well as their high failure rate.
Johnson & Johnson has faced more than 10,000 metal-on-metal hip implant lawsuits from all over the country, including coordinated cases in California state court and a multidistrict litigation trial in Ohio state court.
DePuy ASR Hip Implant Settlements
Johnson & Johnson subsidiary DePuy Orthopaedics previously agreed to offer $4 billion to settle thousands of lawsuits in state and federal courts alleging the company sold metal-on-metal hip implants that are prone to premature failure, including the ASR hip implant.
Under the deal, each claimant was projected to receive about $350,000 on average. Other factors like age and medical condition of the patient were reportedly considered when making the final offer.
Between 7,000 to 8,000 DePuy hip lawsuits were settled with the agreement. One of the consistent claims from DePuy hip implant users is that the device allegedly limited the patient’s mobility and caused severe pain. Lawsuits also alleged that debris from the chromium and cobalt device caused tissue death and increased metal ions in the bloodstream.
The metal-on-metal hip implant is expected to last for around 10 to 20 years. However, it was determined that 1 out of 8 of those who received the device had to have it replaced in the first 5 years.
Another settlement was reached in January 2019 to resolve claims that the ASR hip implant is defective. DePuy and its parent company Johnson & Johnson will pay $120 million as a part of their agreement with 46 attorneys general in the United States. The settlement also required DePuy to maintain a post-market surveillance program that will help track outcomes of ASR patients and provide an accurate idea of how many of these patients need revision surgery due to implant failure.
Although this is not the first substantial settlement reached by the company, DePuy said that they admit no wrongdoing or liability as part of the settlement.
“DePuy Synthes remains committed to meeting the current and future needs of orthopedic surgeons and patients,” the company said in a statement.
Individuals who take legal action against DePuy may be able to benefit from settlements such as these. Legal professionals estimate that metal hip implant awards should range between $200,000 and $500,000 per patient based on past settlements and other device litigation.
Join a Free Metal Hip Implant Revision Surgery Lawsuit Investigation
If you or someone you know has or needs to have your metal on metal hip implant replaced because of any of a number of complications, a hip implant attorney would like to speak with you to determine if you are owed compensation for your injuries.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Metal Hip Implant Revision Surgery Lawsuit Investigation
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.
Oops! We could not locate your form.