Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.

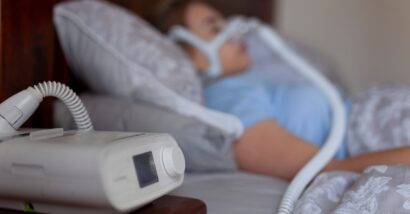
In June, Philips North America LLC recalled millions of its BiPAP breathing and CPAP machines that contain toxic foam, leading to dozens of class action lawsuits claiming that the company initially hid the problem from consumers, and is now forcing them to purchase new devices.
Between three and four million of the Bi-Level Positive Airway Pressure (BiPAP), Continuous Positive Airway Pressure (CPAP), and mechanical ventilator devices were recalled by Philips and its associated manufacturers on June 14, 2021. The BiPAP and CPAP machine recall affected devices in the first-generation DreamStation product family that contain PE-PUR sound abatement foam, including:
- E30 (Emergency Use Authorization)
- DreamStation ASV
- DreamStation ST, AVAPS
- SystemOne ASV4
- C Series ASV, S/T, AVAPS
- OmniLab Advanced Plus In-Lab Titration Device
- SystemOne (Q series)
- DreamStation CPAP, Auto CPAP, BiPAP
- DreamStation GO CPAP, APAP
- Dorma 400, 500 CPAP
- REMStar SE Auto CPAP
- Trilogy 100 Ventilator
- Trilogy 200 Ventilator
- Garbin Plus, Aeris, LifeVent Ventilator
- A-Series BiPAP V30 Auto Ventilator
The company warned consumers to stop using the recalled BiPAP and CPAP machines because the foam was found to degrade into particles that could be inhaled by the user. Toxins in the foam have been linked to “headache, irritation, hypersensitivity, nausea/vomiting, and possible toxic and carcinogenic effects,” warned the recall notice.
Philips acknowledged that some users rely on these life-sustaining devices and could not stop using them without risking severe injury or even death. The company also promised to replace the sound abatement foam as soon as possible, but some BiPAP and CPAP machine users say Philips is not moving fast enough.
In the month since the BiPAP and CPAP machine recall, dozens of class action lawsuits have been lodged against the company in multiple states by consumers who say Philips knowingly exposed them and others to dangerous toxins present in sound muffling foam included in their CPAP and BiPAP machines.
In addition, the plaintiffs allege that despite the fact that patients use BiPAP and CPAP machine on a daily basis to cope with life-threatening conditions, Philips has announced “no concrete timeline for replacing or repairing” the recalled devices.
At least one class action lawsuit further points out that the timing of the CPAP mask recall coincides with the introduction of the next generation of devices by the company — forcing users to buy new Philips CPAP and BiPAP machines.
On Tuesday, one CPAP mask class action lawsuit filed in Pennsylvania federal court was dismissed after the plaintiff and Philips were sent to an Alternative Dispute Resolution process.
Top Class Actions will continue to monitor the Philips CPAP machine recall and related class action lawsuits. Check back for updates!
Do you use a recalled BiPAP and CPAP machine? We want to hear from you! Tell us about your situation in the comment section below or see if you qualify for a Philips CPAP machine recall lawsuit!
The Philips CPAP Class Action Lawsuit sent to arbitration is Thomas v. Koninklijke Philips N.V., et al., Case No. 2:21-CV-00874-CCW in the U.S. District Court for the District of Pennsylvania.
Don’t Miss Out!
Check out our list of Class Action Lawsuits and Class Action Settlements you may qualify to join!
Read About More Class Action Lawsuits & Class Action Settlements:
- Sleep Apnea Sufferers Sue Philips for Making Ventilator With ‘Carcinogenic’ Defect
- Philips Knew of CPAP Problems Before Recall, Claim Class Action Lawsuits
- Do You Qualify: Philips CPAP Machine Recall Lawsuit Investigation
- Philips Endangered Consumers With Its Sleep and Respiratory Care Devices, Class Action Alleges














118 thoughts onCPAP Recap: Consumers Launch Class Actions After Philips ‘Toxic’ BiPAP and CPAP Machines Recall
I have one of the recalled CPAP machines (DreamStation). I have registered it with Philips. They have acknowledged that my machine is one of the devices that is included in the recall. Now I sit and wait to see what they are going to do. I have been experiencing respiratory distress and am being followed by a Pulmonologist.
I am currently using a DreamStation BiPap machine since 2016. Please add me.
I still haven’t heard anything since I reported mine to Phillips. Has anyone else heard anything?
Please add me
I have a qualified serial number machine so add me
I have a Cpap machine. And have had the same one for years. Please add
Add me
Add me
Add me
Please add me
If you can read, there is a process to go through. They won’t just add people at their request. Wake up people.
please add me.