 While the most common infection heater cooler devices have been linked with is mycobacteria chimaera, it may not be the only such infection.
While the most common infection heater cooler devices have been linked with is mycobacteria chimaera, it may not be the only such infection.
A number of heater cooler devices have been reportedly connected with contamination and infection during open-chest surgery.
Around one million U.S. patients may have gone through open-chest surgeries since 2011 while one of these potentially contaminated heater cooler devices was in use, opening them up to the possibility of serious infection.
The risk of contamination and infection associated with heater cooler devices has been a public concern for some time.
Both the U.S. Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) have been releasing information on this connection since October 2015, alerting the public to the potential for serious infection linked with heater cooler devices.
These organizations warned the public that heater cooler devices had the potential to transmit life-threatening bacterial infections to patients during open-chest surgery, specifically surgeries that used cardiopulmonary bypass.
The information released by the FDA and CDC was general, not naming any particular device or manufacturer. The warning also did not disclose any particular type of infection.
Heater Cooler Devices
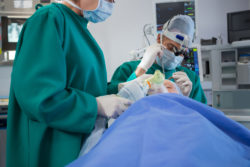
Cardiac heater cooler devices are intended to adjust and control the temperature at which an open-chest surgery patient’s blood is kept. These surgeries include heart valve repair and replacement, heart transplant and heart-lung transplant, and congenital heart abnormality repair.
While neither the FDA nor the CDC specified a particular kind of device or infection, the Stockert 3T heater cooler from LivaNova has become the subject of more recent concern. The Stocker 3T device is associated specifically with mycobacterium chimaera infection.
Stocker 3T heater cooler devices have, in recent months, been singled out in FDA reports as a significant risk factor for mycobacterium chimaera infection after open-chest surgery.
An FDA report noted that the Stockert 3T model may have become contaminated during manufacturing, meaning that other Stockert 3T device may be similarly contaminated, placing patients across the country at significant risk of infection.
According to the FDA report, LivaNova has responded to this reported contamination by instigating cleaning and disinfection processes on its heater cooler devices.
The CDC has also issued an alert about this danger, singling out the Stockert 3T model out of all heater cooler devices as a risk for mycobacterium chimaera infection. The CDC notes that “it is imperative that patients and providers are informed about the risk of infection associated with the use of the 3T device.”
While these federal notices have singled out specific heater cooler devices and potential infection problems, some researchers note that this does not mean that other heater cooler devices do not cause infection as well, or that mycobacterim chimaera is the only potential infection.
Even though mycobacterium chimaera is linked with the majority of infections from heater cooler devices, other potentially minor to life-threatening infections could be possible.
If you or someone you love has suffered from an open chest surgery infection such as mycobacterium chimaera after undergoing surgery with the use of a Sorin 3T System or similar device, you may be able to file a lawsuit or join a class action lawsuit.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The hip implant attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. [In general, metal hip implant lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Cardiac Heater-Cooler System Class Action Lawsuit Investigation
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Please Note: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client, if you qualify, or getting you dropped as a client.
Email any problems with this form to [email protected].
Oops! We could not locate your form.












