NFH iron supplement recall overview:
- Who: Nutritional Fundamentals for Health (NFH) is recalling approximately 17,660 bottles of its iron dietary supplements.
- Why: The packaging of these supplements is not child-resistant, posing a risk of poisoning if ingested by young children.
- Where: The recall is effective across the United States and Canada.
Nutritional Fundamentals for Health has issued a recall for more than 17,000 bottles of its iron dietary supplements due to packaging that fails to meet child safety standards.
The NFH recall was announced on March 27 for bottles that are not child-resistant as required by the Poison Prevention Packaging Act (PPPA).
This oversight poses a significant poisoning risk for children who may access the supplements.
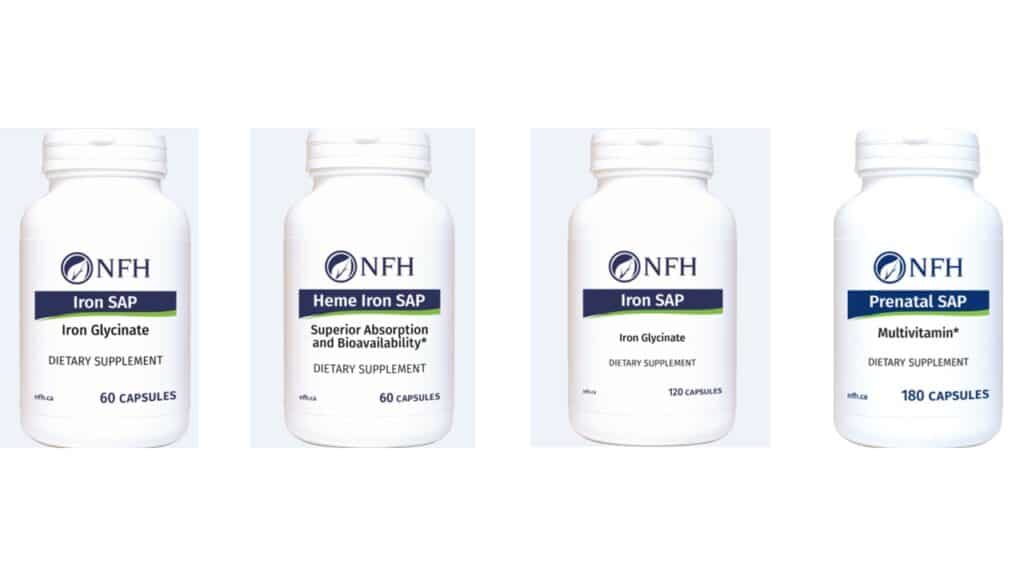
The NFH recall affects NFH Iron SAP, NFH Heme Iron SAP and NFH Prenatal SAP bottles sold nationwide and online between March 2022 and December 2024.
According to the recall notice, the affected products include various sizes and date codes, such as Iron SAP with 60 and 120 capsules, Heme Iron SAP with 60 capsules and Prenatal SAP with 180 capsules.
NFH recall urges consumers to return supplement bottles
“Consumers should immediately secure the recalled supplement bottles out of sight and reach of children,” the recall advises. NFH is actively reaching out to known purchasers to facilitate the replacement of the non-compliant bottles with child-resistant ones.
The supplements were sold through multiple naturopathic and homeopathic clinics as well as online platforms like WholescriptsInc.com and Fullscript.com. Prices ranged from $20 to $95, depending on the product and size.
Consumers who have the products should discontinue use immediately and return it to the place of purchase or discard it, the recall notice says. The supplements were manufactured in Canada and imported by NFH, also based in Canada.
Consumers can obtain more information about the recall by visiting NFH’s website, calling the NFH’s toll-free number 866-510-3123 or emailing [email protected].
NFH reports that there have been no incidents or injuries associated with the recalled products so far. The company is not currently facing any legal action over the recall, but such recalls can sometimes lead to class action lawsuits, which are closely monitored by Top Class Actions.
In 2022, Rae Wellness recalled its Rae Prenatal Capsules and Rae Immunity Capsules because the products were not sold in child-safe packaging, posing a risk of poisoning in children.
Are you affected by the NFH recall? Let us know in the comments.
Don’t Miss Out!
Check out our list of Class Action Lawsuits and Class Action Settlements you may qualify to join!
Read About More Class Action Lawsuits & Class Action Settlements:















10 thoughts onNFH recalls iron supplements due to child poisoning risk
Add me pl
please add me
Add me
Add me please
Please add me.
Add me please
add me
Add me !
Please add me
please add me