Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
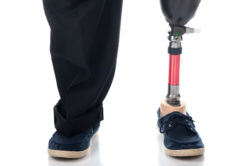
Invokana, a medication used to treat type-2 diabetes, is part of a class of drugs known as sodium-glucose cotransporter-2 (SGLT2) inhibitors. A growing number of patients report problems with Invokana limb amputation.
Also included in this class are Jardiance, Farxiga, Invokamet, and Invokamet XR. The latter two contain the same active ingredient as Invokana—canaglifozin—with metformin, a more traditional diabetic medication.
The FDA is requiring a label change of Invokana, Invokamet, and Invokamet XR that includes a black box warning. Black box label warnings are reserved for drugs with the potential for very serious adverse side effects such as Invokana limb amputation. Recent clinical trials were completed that had monitored patients taking Invokana, Invokamet and Invokamet XR against a control group taking a pill without an active ingredient.
Upon conclusion of these trials and configuration of the results, it was found that those ingesting the SGLT2 inhibitors were twice as likely to have had to undergo Invokana limb amputation of toes, feet, and legs. The FDA sent out a safety announcement in May 2017 and posted the trial conclusions on its website.
Certain patients taking Invokana, Invokamet, and Invokamet XR have an increased risk of needing Invokana limb amputation by virtue of medical events and conditions existing prior to starting treatment. These medical conditions include neuropathy, peripheral vascular disease, previous amputations, and ulcerative conditions of the foot.
Early signs that a patient might be developing a problem that could lead to Invokana limb amputation might be new infections or ulcers in the feet, pain, and swelling. Patients that have been taking this drug should consult their physician if any of these symptoms are noticed.
In addition to Invokana limb amputation, Invokana, Invokamet, and Invokamet XR have been implicated in other life-threatening conditions leading to over two hundred filed lawsuits against the pharmaceutical giant, Johnson & Johnson. One of these serious alleged conditions is kidney failure.
Invokana works by inhibiting the body from reabsorbing extra glucose in the blood and directing it out through the body’s urine output. While a good idea theoretically, this inhibition of the reabsorption process puts extra strain on the kidneys which ultimately may prove dangerous in a certain percentage of takers.
Ketoacidosis, a condition in which the body’s fluids become highly acidic, has also been linked to taking SGLT2 inhibitors such as Invokana. The condition is created by the body producing acids known as ketones in excess. This often happens when a person goes a lengthy period of time without ingesting food or when insulin levels drop precipitously.
The relationship of SGLT2 inhibitors to ketoacidosis was warned about by the FDA in May 2015 in a separate safety announcement. Symptoms of ketoacidosis can include upset stomach, shortness of breath, throwing up, unexplained exhaustion, and a lack of mental clarity.
In general, Invokana and Invokamet lawsuits are filed individually by each plaintiff and are not class actions.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Invokana Class Action Lawsuit Investigation
If you or a loved one suffered ketoacidosis or lower extremity amputation after taking Invokana, Invokamet, or Invokamet XR, you may have a legal claim. See if you qualify to pursue compensation and join a free diabetes medication class action lawsuit investigation by submitting your information for a free case evaluation.
An attorney will contact you if you qualify to discuss the details of your potential case.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
Oops! We could not locate your form.












