Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
Uloric (febuxostat) outcomes were compared with those of allopurinol in a clinical trial presented at the American College of Cardiology’s Annual Scientific Session in March 2018. Results of the CARES trial (Cardiovascular Safety of Febuxostat and Allopurinol in Patients with Gout and Cardiovascular Morbidities) also were published in the New England Journal of Medicine by the study’s lead investigator and author William B. White, MD.
Even though Uloric gout medicine showed an increased risk of heart-related death, Uloric and allopurinol appear to have similar results for nonfatal heart attacks and nonfatal strokes.
The CARES trial looked at 6,000 gout patients between 2010 and 2017. They all had gout and some type of history of cardiovascular disease.
Why Uloric apparently causes an increased risk of sudden cardiac deaths is uncertain, according to the report. This is reportedly the first time a cardiovascular study has shown an increased risk of cardiac death without also indicating an increased risk of nonfatal heart attacks and nonfatal strokes.
The CARES trial was funded by Takeda Pharmaceuticals, maker of Uloric.
Why Patients take Uloric Gout Medicine
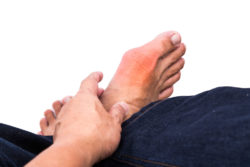
Gout is a type of inflammatory arthritis caused by too much uric acid in the blood. The uric acid crystallizes and settles into the joints. Most often, a big toe is the first area to experience a gout attack, marked by severe pain, swelling and redness.
Even though diet and exercise can help prevent gout attacks, some of the 8 million Americans suffering from gout reportedly need medicine to help prevent uric acid accumulation.
Uloric gout medicine was approved by the FDA in 2009. Its main competitor, allopurinol, was FDA-approved in 1966. They are in a class of drugs known as xanthine oxidase inhibitors.
The CARES clinical trial was completed as an FDA requirement. Upon learning the results, the FDA released two Drug Safety Communications. The initial communication indicated the FDA would evaluate the increased risk of cardiac-related deaths seen in Uloric patients. The second communication said the FDA would continue evaluating not only cardiac-related deaths, but also deaths from all causes among those who took Uloric
“Health care professionals should consider this safety information when deciding whether to prescribe or continue patients on febuxostat,” the FDA stated. “Patients should talk to your health care professionals if you have any questions or concerns. Do not stop taking your medicine without first consulting with your health care professional.”
According to Uloric’s website, Uloric gout medicine can cause gout to flare up when first starting the medication. Other side effects can include liver problems. Serious skin and allergic reactions might adversely affect the liver, kidneys, heart, or lungs.
A rare, severe allergic reaction known as Stevens Johnson Syndrome (SJS) has also been reportedly linked to Uloric gout medicine. The condition can become life-threatening. SJS starts out as a rash but quickly escalates to include painful blisters appearing on the mucus membranes, swollen face, lips, mouth, tongue or throat, and symptoms similar to the flu.
In general, Uloric lawsuits are filed individually by each plaintiff and are not class actions.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual Uloric lawsuit or Uloric class action lawsuit is best for you. Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Uloric Class Action Lawsuit Investigation
If you suffered from a serious side effect or a loved one died while taking Uloric, you may have a legal claim. See if you qualify to pursue compensation and join a free Uloric lawsuit investigation by submitting your information for a free case evaluation.
An attorney will contact you if you qualify to discuss the details of your potential case.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
Oops! We could not locate your form.












