Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
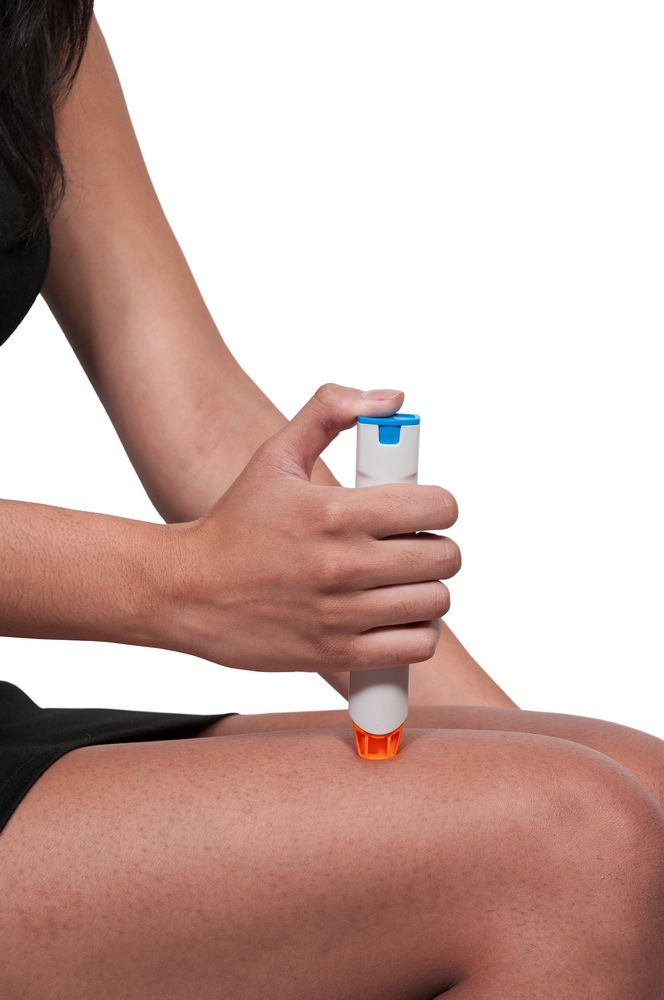
The plaintiffs specifically challenge Mylan’s practice of marketing EpiPens exclusively in two-injector packs rather than as single injectors.
They claim Mylan uses the two-pack marketing solely to boost its revenues, requiring persons who depend on EpiPen for their life and health to purchase more of it.
They accuse Mylan of misstating the available scientific evidence to justify the two-pack.
These alleged misstatements violate the consumer protection laws of the named plaintiffs’ home states and those of several other states, they argue.
EpiPen is a device that uses a spring-loaded needle to inject a single dose of epinephrine, or adrenaline. It’s used as a first-line treatment for anaphylaxis, a severe, potentially life-threatening allergic reaction.
Persons with severe allergies who are at risk for anaphylaxis are advised to keep EpiPen handy at all times for emergency use, according to the class action lawsuit.
Mylan has held worldwide rights to EpiPen when it acquired Dey Pharma in 2007.
According to the plaintiffs, Mylan’s EpiPen now controls an 85 percent share of the epinephrine autoinjector market, a market reportedly worth $1.3 billion. Since acquiring the rights to EpiPen, Mylan has increased its wholesale price by about 400 percent, the plaintiffs say.
When Mylan announced that it would cease marketing EpiPen as a single injector, it cited as justification a 2010 report by the National Institute of Allergy and Infectious Diseases.
Plaintiffs say that report provided only “limited information concerning the possible need for additional doses of epinephrine.”
The EpiPen class action lawsuit further claims Mylan grossly overstated its medical justification for the two-pack.
Plaintiffs say that one of the studies Mylan cited shows that only a small proportion of persons required a second dose, and almost all of those received their second dose from a health care professional.
Plaintiffs also say no study shows any significant benefit would come from forcing physicians to prescribe two EpiPen injectors at a time, as opposed to leaving them the option of prescribing only one at a time.
They accuse Mylan of taking away physicians’ judgment by discontinuing the single-injector option.
The plaintiffs are proposing a nationwide plaintiff Class consisting of all EpiPen purchasers who were required to purchase their EpiPens in two-packs or would in the future be required to do so in the absence of the relief requested in this EpiPen class action lawsuit.
They are also proposing four subclasses consisting of Class Members from each of the named plaintiffs’ home states –specifically, Michigan, Kentucky, Connecticut and New Hampshire.
They are asking the court to certify the proposed Class and to award them damages, restitution and disgorgement of profits, attorneys’ fees and costs of litigation. They also seek an injunction barring Mylan from limiting sales of EpiPen to packs of two and from charging unconscionable prices for EpiPen.
Representing the plaintiffs are attorneys Sharon S. Almonrode, E. Powell Miller, Christopher D. Kaye and Mahde Y. Abdallah of The Miller Law Firm PC, and Ari Kresch of Excolo Law PLLC.
The EpiPen Deceptive Practices Class Action Lawsuit is Anastasia Johnston, et al. v. Mylan Specialty L.P., et al., Case No. 2:16-cv-13060, in the U.S. District Court for the Eastern District of Michigan.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.















42 thoughts onEpiPen Class Action Says Two-Packs Violate Consumer Protection Laws
When do we get an up date please? I’ve received no letters emails or updates like the last settlement I was involved with.
What the update on this?
Any update on this and how do i get involved. I use epi pens
Is there any update to this. Im very interested. I use epi pens
I used epipens for server anaphylaxis from bees and raw tomatoes. Because of the price I only keep a script om file…this will not help in an emergency. However I found out it is the “auto- injector” they claim is copywrited and costs so much. I now get my epinephrine in a vile and use 0.4 mg in a needle ready to go. This is not the best practice but the auto injector is 20x,s the cost of the adrenalin drug