Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
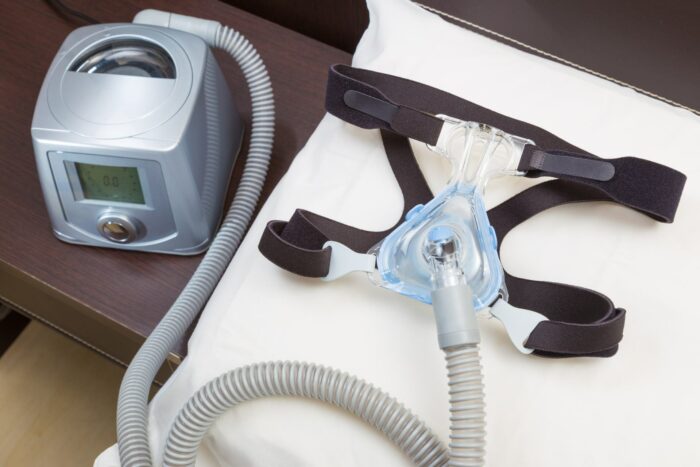
Philips is recalling millions of its CPAP machines, ventilators, and other breathing devices – many used for sleep apnea – due to potential risks of chemical exposure posed by noise-reducing foam in the devices made from polyester-based polyurethane.
Between three and four million of the Bi-Level Positive Airway Pressure (Bi-Level PAP), Continuous Positive Airway Pressure (CPAP), and mechanical ventilator devices are being recalled.
The company has not received any reports of death connected to the sound abatement foam or any reports of impact from chemical emissions, but it has received reports of possible patient impact due to foam degradation, it says in the recall notice.
“The potential risks of particulate exposure include headache, irritation, inflammation, respiratory issues, and possible toxic and carcinogenic effects. The potential risks of chemical exposure due to off-gassing include headache, irritation, hypersensitivity, nausea/vomiting, and possible toxic and carcinogenic effects.”
Despite a low complaint rate (0.03 percent in 2020), testing by the company determined that there were possible risks to users related to this type of foam, including that it might degrade into particles. Philips says in the notice that those particles may enter the device’s air pathway and be ingested or inhaled by the user, and the foam may off-gas certain chemicals.
“The foam degradation may be exacerbated by use of unapproved cleaning methods, such as ozone, and high heat and high humidity environments may also contribute to foam degradation,” the company says.
Frans van Houten, CEO of Royal Philips said the company deeply regrets any concern and inconvenience caused to patients using the affected devices.
“In consultation with the relevant regulatory agencies and in close collaboration with our customers and partners, we are working hard towards a resolution, which includes the deployment of the updated instructions for use and a comprehensive repair and replacement program for the affected devices. Patient safety is at the heart of everything we do at Philips.”
The company is urging patients using BiLevel PAP and CPAP devices to discontinue use and to work with their physician to determine the most appropriate options for continued treatment.
Philips says that patients using life-sustaining mechanical ventilator devices should not stop or alter their prescribed therapy until they have talked to their physician.
“Philips recognizes that alternate ventilator options for therapy may not exist or may be severely limited for patients who require a ventilator for life-sustaining therapy, or in cases where therapy disruption is unacceptable,” the company says in the recall notice.
“In these situations, and at the discretion of the treating clinical team, the benefit of continued usage of these ventilator devices may outweigh the risks identified in the recall notification.”
Philips says that it will replace the current sound abatement foam with a new material and has already begun the preparations, which include getting the relevant regulatory clearances. It says that it aims to address all affected devices in scope of this correction as expeditiously as possible.
“As part of the program, the first-generation DreamStation product families will be modified with a different sound abatement foam and shipped upon receipt of the required regulatory clearances. Philips’ recently launched next-generation CPAP platform, DreamStation 2, is not affected by the issue.”
To support the program, Philips is increasing the production of its DreamStation 2 CPAP devices that are available in the US and selected countries in Europe.
For more information on the recall notification, as well as instructions for customers, users and physicians, affected parties may contact their local Philips representative or visit www.philips.com/SRC-updateExternal Link Disclaimer.
Have you used a breathing device manufactured by Philips? How do you feel about this recall? Let us know in the comments section or join a CPAP class action lawsuit investigation!
Don’t Miss Out!
Check out our list of Class Action Lawsuits and Class Action Settlements you may qualify to join!
Read About More Class Action Lawsuits & Class Action Settlements:
- Consumer Protection Agency Announces New Rules for Dangerous Infant Sleepers
- Do You Qualify: Asbestos Cancer | Mesothelioma and Lung Cancer Lawsuit Claim Review
- Recall Check: Fisher-Price Recalls Gliders After Four Infant Deaths
- Do You Qualify: Surgical Staples Complications and Recall Lawsuit Investigation













117 thoughts onRecall Check: Philips Recalls Millions of CPAP and Ventilators Over Fears of Chemical Exposure, Carcinogenic Effects
I’ve been using the Dreamstation for a little over a year and now this. I’ve contacted P-R several times, registered my device, but they can’t say when the new pieces will be shipped out. They just say “We regret to inform you that it will take a whle.” What the hell is that? What kind of accountability is that? And the website says nothing. But you better believe you have to listen to 2 minutes or legal disclaimers now before you even get to speak to someone. They know a class action is coming.
I’ve been using the Phillips Dreamstation for about 14 months and have started having a lot of sinus headaches. I will not use this machine again. I have asked to return the machine to Coastal Medical Supply and have asked my doctor to start service with another vendor using a different machine. I’m not waiting around for the refurbished machine that may still be contaminated with dust.
I’m livid about this. Have been on the phone on and off for hours today and got nowhere. I have pretty severe opstructive sleep apnea, so not using my CPAP is not really feasible. Thus, I am basically left with continuing to use my CPAP with the known risks, or stop using and constantly waking up in the middle of the night because I stop breathing.
I spoke to my CPAP supplier, and they flat-out said they’ve been given no guidance, and it is not their responsibility and to contact my doctor. He compared the recall to that of a car recall – you may have bought the car at the dealership (i.e. my CPAP supplier), but the manufacturer deals with the recall. I reminded him that there’s always an option to stop driving your car until the issue can be fixed, you can’t really do that with a necessary medical device. He agreed, but was of no help otherwise.
I actually spoke directly with a Philips representative, and while he was very nice and understanding, he was completely unhelpful as Philips still hasn’t given any guidance or indication as to when they will get this resolved. Which is just absurd considering they initiated the recall a week ago! What company initiates a recall without any plans on how to actually do a recall.
To make an already long story slightly less long, Philips has opened themselves up to some major lawsuits here, and I absolutely will be getting involved. This situation is completely unacceptable.
i have used the cpaps around 13 years now. i have heartfailure lung cancer had skin cancer removed headaches and sick belly
Please include me
I have had constant headaches for about a year. They have adjusted my dream station twice and still the same… some turn into migraines. Horrible pressure and burning in my sinuses
I’ve had my Dreamstation since July 24, 2017. All through this time I’ve had ear, sinus, and throat infections which never happened before I started the CPAP therapy.
In January 2021 I sent my machine in for repair; they replaced a few gaskets between the humidifier and the CPAP and fixed the clasp for the humidifier door.
Since I received my repaired machine, around January 27, I have had air pushed into my stomach and have awakened in the night feeling pressure in my throat as though I had a burp stuck in my throat. Obstructive Apnea.
Memorial Day Weekend I woke up Saturday and Sunday feeling like an overinflated balloon. I had air in my stomach Saturday; Sunday I had air in my stomach and all the way through to my rectum. I was in extreme pain and I was running a fever of 102.
I thought I had COVID, but test results were negative.
I spoke to a Philips Respironics Customer Service Representative on June 14, before seeing my doctor, and she asked me if I thought I had been damaged by this experience. RED FLAG. I told her I don’t know and that is why I’m going to my doctor.
I have 30 known chemical intolerances and I want a list of all the chemicals I’ve been exposed to.
I also demand a brand new machine, a Dreamstation 2, not a repair or refurb as they are promising on their registration and information websites. I will NOT expose myself to those chemicals again. I also want new hose, frame, masks, filters, and tank as the items I have are contaminated.
I’ve not used my machine since Sunday, May 30. When I spoke to my DME Provider, he suggested I get an attorney.
Before anyone can be tested for exposure, or the extent of the exposure; we have to know what chemicals we are dealing with. I’ve not seen a list, has anyone else?
Compared to the cancer reports on this page, my body’s response has been mild, but for my body, this is not mild. My lungs are tight and my breathing during the day is back to pre-CPAP shallowness and I’m always feeling over exerted; and my sleep is terrible.
If there is an actual class action lawsuit in the works, sign me up.
Repair or Refurb…oh hellz no!
I have used two recalled machines for a total of over 5 years. I developed melanoma, had cataract surgery and developed swelling of the optic nerve which left my right eye Vision impaired. Had MRI trying to determine why and discovered two areas of white matter that has yet to be determined what it is. I have had a terrible cough for the last couple of years where I cough until I through up. I cannot find a doctor in my area that follows through on my health. Now I am scared it is all related to this. I want tested for everything possible this could have caused. Not sure I want to use another machine
So the recall states possible carcinogenic effects, what happens if you just had lung cancer and had to half the right lung removed? The site FAQ doesn’t give any info for how to proceed, who to file a claim with, or what happens in this situation.
Is this a different claim process?
The same thing happened to my wife. 18 months after beginning use cancerous nodules were discover and 3 months later a lobectomy. We are currently seeking council.
Contact an attorney asap
Wow! I’m just floored. I’ve been using the Dreamstation for about 2 years and what started with a bad cough during Covid so I couldn’t get in to see my doctor has turned in to a May 4th diagnosis of Non Small Cell Squamous Lung Cancer! I guess I better speak to an attorney.
I have been using the philips dreamstation since Dec 2020. After only a few weeks use my sinus problems were terrible. Then about a month ago, along with the upper respiratory misery, my lungs began to feel congested. I have mild apnea but my cardiologist said that cpap therapy might help my AFib ( it doesn’t). I refuse to use this thing another night and just hope that the problems it might have caused will clear on their own.