Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
The side effects of Elmiron (pentosan polysulfate, or PPS) include gastrointestinal problems, like diarrhea and heartburn. However, some patients have reported suffering from a much more serious side effect involving eye damage that includes macular degeneration, after taking the drug.
What Is Elmiron?
Elmiron is an oral medication typically used to treat the bladder disorder interstitial cystitis. It is what is known as a semi-synthetic polysulfated xylan, derived from xylan, a substance found in the bark of the beech tree. This substance is then treated with sulfating agents, similar to those used in food preservatives, in order to produce the medicine.
Why Is Elmiron Prescribed?
Primarily, Elmiron is indicated for the treatment of interstitial cystitis, also known as “bladder pain syndrome” (BPS). Interstitial cystitis is a chronic and painful condition that may result in extreme pelvic pain, as well as a frequent and persistent need to urinate. There is also research to suggest that it may be effective for treating osteoarthritis of the knee, which occurs when joint cartilage and the surrounding bone begins to break down.
It is unknown exactly how Elmiron works. Medical scientists believe that, because of Elmiron’s similarity to the bladder wall’s natural lining (which is damaged in patients with interstitial cystitis), it is able to effect healing and repairs. The drug may work by restoring or coating the inner surface of the bladder, which protects the organ from irritation or inflammation caused by waste products in the urine.
What Are Side Effects of Elmiron?
In general, medications typically do come with a list of certain mild side effects. But some medications have more chronic or severe side effects than others. In some cases, drug manufacturers may not have adequately warned about a particular health complication.
Most patients who suffer Elmiron side effects complain of gastrointestinal problems, including diarrhea, indigestion, nausea, abdominal pain, and heartburn. Others report suffering from insomnia, headaches, hair loss, and skin rashes. According to approval documents from the U.S. Food and Drug Administration (FDA), these events occurred in between 1% to 4% of patients.
Because Elmiron has an anticoagulant effect (prevents blood clotting), patients taking the drug may bruise more easily. For this reason, patients who are scheduled for major surgery are told to stop taking the medication beforehand.
Other Elmiron side effects occurred in less than 1% of patients. This includes allergic reactions, vomiting, anemia, and more.
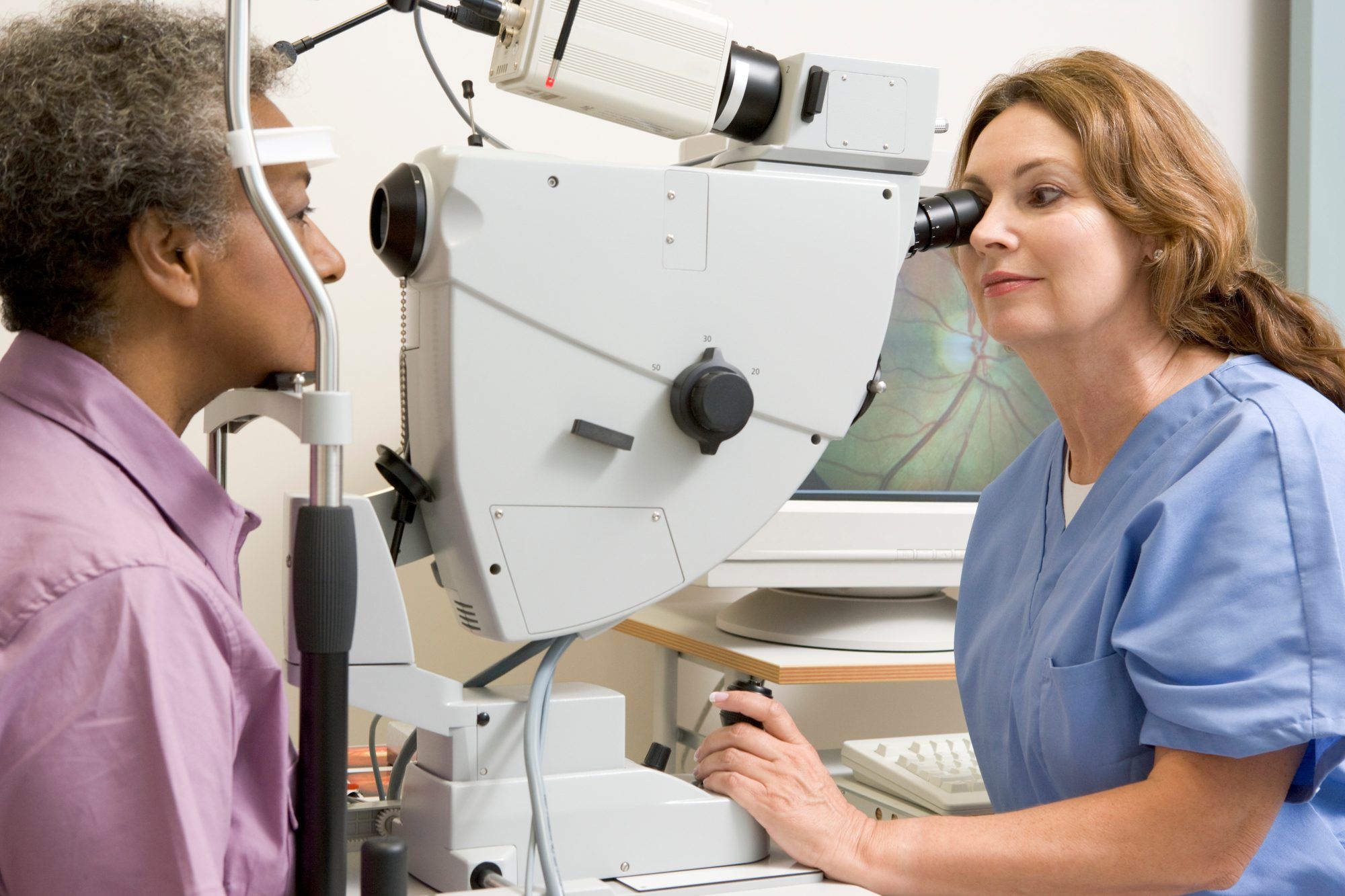
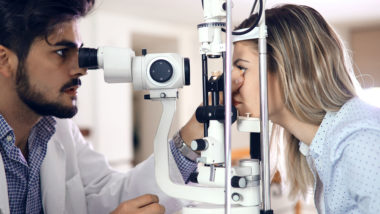
Additionally, some patients have reported suffering from vision problems after taking Elmiron, including a severe condition called pigmentary maculopathy.
This condition is also known as maculopathy, or macular degeneration. It is a gradual, progressive degenerative condition that causes loss of central vision first, and can eventually cause blindness.
Although age-related maculopathy is a common condition affecting approximately 11 million older people in the U.S., maculopathy caused by Elmiron exposure may affect people of any age.
There are several types of maculopathy. The type that has been associated with Elmiron is known as pigmentary maculopathy, which tends to occur after a long period of Elmiron therapy. When this happens, the pigmented cells underneath the retina break down, releasing pigment that winds up settling on the macula. The result can be vision distortion and/or blurriness, difficulty adjusting to light and darkness, and loss of vision.
According to reports, it can take years for eye damage from Elmiron exposure to develop.
Is Pigmentary Maculopathy Reversible?
While clinical research has found that Elmiron causes structural changes to the retina’s pigmented layer, it is not known if stopping the medication will result in a reversal of the condition. Unfortunately, some patients claim that they continue to experience vision loss or eye problems years after they ceased taking Elmiron, with some claiming that their condition has continued to deteriorate.
What Are the Symptoms of Pigmentary Maculopathy?
Pigmentary maculopathy may cause symptoms including a dark spot in the field of vision, vision distortion, or hallucinations including flashing lights or loss of sensitivity to color or contrast. Signs of this condition include eye pain, blurred vision, trouble adjusting to the darkness, trouble reading, and vision loss.

What Are the Chances of Developing Pigmentary Maculopathy?
Kaiser Permanente ophthalmologists Robin A. Vora, M.D., Amar P. Patel, M.D., and Ronald Melles, M.D. reportedly looked back at patient records of 4.3 million patients. They decided to delve into those records after one of their patients who had been taking Elmiron for a long time was misdiagnosed with retinal dystrophy instead of pigmentary maculopathy.
The doctors identified 140 patients who each had been prescribed about 5,000 Elmiron pills over a 15-year timeline, according to Science Daily. Of those 140 patients, 91 said they would return for an updated eye exam.
They found 22 of the 91 patients had indications of drug toxicity, which was linked to the amount of Elmiron they had taken.
Eleven percent of patients on 500 to 1,000 grams of Elmiron showed toxicity, but 42 percent of patients taking 1,500 grams or more showed clear signs of Elmiron toxicity.
The amount of Elmiron that could result in toxicity is unknown, which prompted Dr. Vora to suggest patients who don’t show signs of drug toxicity to see their eye doctor and have their retinas checked for damage annually. He said anyone with indications of such side effects of Elmiron should then speak to either the urologist or ob/gyn who prescribed the medication and perhaps discuss stopping the regimen.
Elmiron Warning Label Updated
On June 16, 2020, the FDA required the drug maker to add “Retinal Pigmentary Changes,” also known as “pigmentary maculopathy,” to Elmiron’s warning labels.
The Elmiron label now says patients should be wary of the following symptoms:
- Increased difficulty reading
- Slow adjustment of the eyes to low or reduced light environments
- Experiencing blurred vision
The warning label also says that physicians should exercise caution when prescribing Elmiron for patients already diagnosed with retinal pigment changes. Further, doctors are urged to re-think use of the drug in the event a patient develops retina changes.
The IC Network, a publication by the Interstitial Cystitis Network (ICN), reports that the warning label change came after a number of studies and publications, as well as a petition to the FDA and numerous patient reports of the condition were made.
In March 2021, additional updates to the Elmiron medication guide were implemented by the FDA. This guide helps inform patients of Elmiron side effects and risks.
What Did the Drug Manufacturer Know?
Some patients who say they developed pigmentary maculopathy after taking Elmiron claim that the drug maker knew or should have known about the side effect, but failed to inform the public.
In November 2018, the American Academy of Ophthalmology published the results of a study linking side effects of Elmiron in patients taking the medication for interstitial cystitis. These results were confirmed in another study released six months later, that found “potentially avoidable retinal degeneration…associated with chronic exposure [to Elmiron].”
Patients who developed eye problems after taking the drug have filed lawsuits against the company claiming that they should have known of the possibility and warned potential patients about the side effects of Elmiron. These Elmiron lawsuits have been organized into multidistrict litigation and transferred to a federal court in New Jersey, according to Law360.
Filing an Elmiron Lawsuit
If you or someone you love has suffered from eye problems after using Elmiron, you may be able to join the growing number of Elmiron lawsuits and pursue compensation.
Filing a lawsuit can be a daunting prospect, so Top Class Actions has laid the groundwork for you by connecting you with an experienced attorney. Consulting an attorney can help you determine if you have a claim, navigate the complexities of litigation, and maximize your potential compensation.
If you were treated with Elmiron and developed vision problems, you may be eligible to participate in this free Elmiron side effects lawsuit investigation. Fill out the form on this page for more information.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Elmiron Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.
Oops! We could not locate your form.