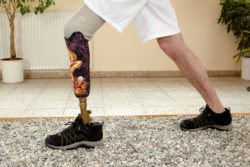
 Invokana and amputations of the lower extremities related to type-2 diabetes have become the subject of a new spate of injury lawsuits. It seems odd that a medication should cause one of the same issues that it was supposedly developed to prevent. However, in retrospect, it is not surprising, considering that prominent medical experts expressed serious doubts about the medication before it even received FDA approval.
Invokana and amputations of the lower extremities related to type-2 diabetes have become the subject of a new spate of injury lawsuits. It seems odd that a medication should cause one of the same issues that it was supposedly developed to prevent. However, in retrospect, it is not surprising, considering that prominent medical experts expressed serious doubts about the medication before it even received FDA approval.
Even though Invokana and a similar medication, Invokamet (a combination of canagliflozin and metformin) have been linked to other health problems that include ketoacidosis and kidney failure, the current Invokana and amputations lawsuit investigation is seeking type-2 diabetics who wound up losing toes, feet and lower limbs after taking the medication.
What is Invokana?
Invokana
(canagliflozin) belongs to a class of diabetic medications known as “SGLT-2 inhibitors,” or gliflozin drugs. These medications operate by preventing the action of a substance in the body known as sodium glucose transporter-2, which is a protein responsible for reabsorption of blood sugar (glucose) in the kidneys.
When this mechanism is disabled, it prevents this reabsorption, allowing excess glucose to be passed in the urine, keeping blood sugar levels at a healthier level. Invokana was originally developed by Japanese pharmaceutical firm Mitsubishi Tanabe, and manufactured and sold in the U.S. by Janssen Pharmaceuticals.
FDA Approval Was Questioned
In January 2013, the FDA Endocrinologic and Metabolic Drugs Advisory Committee (EMDA) held a meeting at which Janssen representatives gave a number of presentations on how its new product, Invokana, would help to address the growing type-2 diabetes epidemic.
During that meeting, Dr. Sidney Wolfe, a prominent physician, noted that Janssen’s claims were “based solely on surrogate efficacy of HbA1c lowering” (referring to the form of hemoglobin that binds to glucose, a common measure of diabetic control). He pointed out that there was “no evidence of any improved clinical outcomes, contrary to an older diabetes drug such as metformin,” and expressed concerns over some of the potential dangers that came up during clinical studies.
Dr. Wolfe was not the only one. Two other medical experts, including FDA reviewer Dr. Hyon Kwon, pointed out that “cardiovascular events” were seen during the clinical trials. Nonetheless, the FDA went ahead and granted approval for Invokana on the premise that the potential benefits outweighed the risks.
The Link Between Invokana and Amputations
Since Invokana’s approval, numerous side effects have been discovered, not the least of which is an association between Invokana and amputations. In May 2017, the FDA issued a safety announcement confirming what had long been suspected: “the type 2 diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR) causes an increased risk of leg and foot amputations.” Since that time, the FDA has required a “black box” warning on all packages, warning of the connection between Invokana and amputations.
These findings on Invokana and amputations were based on the final results from the original clinical studies. These demonstrated that approximately seven out of every 1,000 patients who take Invokana will wind up losing a foot or a leg.
Contact an experienced Invokana attorney today to discuss your legal options if you or a loved one had to undergo a lower limb amputation after taking Invokana, Invokamet or Invokamet XR, including a toe amputation, foot amputation, knee amputation or leg amputation. Fill out the form on this page to obtain a FREE case evaluation with an Invokana amputation attorney.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Invokana Class Action Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
Please Note: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
[email protected].
Oops! We could not locate your form.












