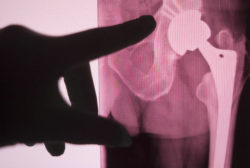
 Recently, Stryker expanded an existing recall of the LFIT V40 femoral head.
Recently, Stryker expanded an existing recall of the LFIT V40 femoral head.
The Stryker LFIT V40 femoral head recall was initiated in August of 2016 following several reports of component failure that could possibly cause patents injury.
Stryker corporation also stated that the hip implant was recalled due to “higher than expected complaints of taper lock failure,” which describes a situation in which the taper lock fails or breaks down, which can include disassociation of the femoral head from the hip stem, fractures, and metal debris being released into the body.
Hip implant recipients who experience taper lock failure are at risk for experiencing pain, inflammation, instability, dislocation, and bone fractures, complications that may require revision surgery to resolve.
The initial recall involved around 42,000 products over concerns that the device could cause injury to patients, and unfortunately, these problems may not be resolved.
In 2018, Stryker expanded the Stryker LFIT V40 femoral head recall, adding eight more catalog numbers to the original seven recalled catalog numbers from 2016. This move in an updated product safety notification that Stryker released on May 22, 2018, warning orthopedic surgeon of possible problems with the device.
Metal Poisoning
Most hip implant devices are designed to have two pieces of differing material fit together. However, the LFIT V40 femoral head was designed to be used with other metal components, meaning that two metal pieces rubbed together in a patient’s body.
This reportedly caused issues because the two metal components could rub improperly together, causing metal fragments to fret off of the device and into the patient’s body. This also allegedly caused a patient to develop elevated metal levels in their blood, and possibly develop infection and irritation. In addition, the functionality of the device was allegedly affected, because as the metal fragments fretted off, the device would not fit together as well as it should, impeding motion.
To add insult to alleged injury, the Stryker LFIT V40 was supposed to minimize the risks typically associated with hip replacement surgery, but may have ended up causing more harm to patients than good. Reportedly, the LFIT V40 device is designed to work with a number of hip implant systems, and was supposed to help minimize the risk of dislocation. However, these devices were reportedly more prone to corrosion.
Stryker has already faced backlash over the LFIT V40, even after the Stryker LFIT V40 femoral head recall. Patients claim the company knew or should have known that the device could have problems and cause injury, but released it onto the market nonetheless.
This problem has raised concerns around the world. In Australia, the Department of Health also alerted consumers to the possible issues associated with the Stryker femoral heads.
The problems around the LFIT V40 femoral head recall isn’t the first time that Stryker has faced backlash over hip products — they have already reportedly paid over $1 billion to settle hip implant lawsuits alleging injury-causing defects in their other hip products.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The hip implant attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. [In general, metal hip implant lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2025 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Stryker Metal Hip Replacement Class Action Lawsuit Investigation
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
Email any problems with this form to [email protected].
Oops! We could not locate your form.